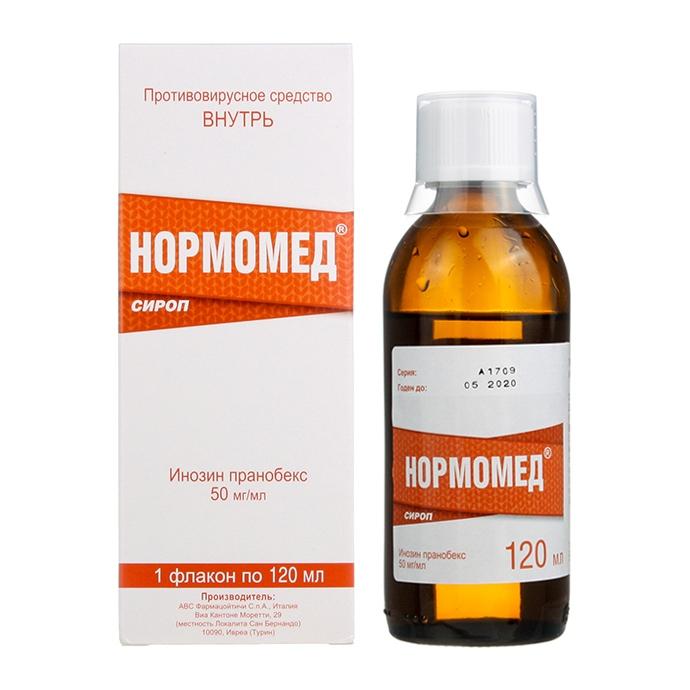
Description
Release form
Syrup.
Indications
Immunodeficiency, caused by viral infections in patients with normal and weakened immune systems, including diseases caused by Herpes simplex viruses types 1 and 2 (including genital herpes and herpes of a different localization), Varicella zoster (shingles, chicken pox) subacute sclerosing panencephalitis.
Treatment of influenza and other acute respiratory viral infections, infectious mononucleosis caused by the Epstein-Barr virus, cytomegalovirus infection, severe measles, papillomavirus infection: laryngeal papillomas / vocal cords (fibrous type), genital papillomavirus infection in men and women, wart contagiosum.
Contraindications
Gout urolithiasis arrhythmia chronic renal failure children under 3 years of age (body weight up to 15-20 kg) pregnancy lactation (breastfeeding) hypersensitivity to the drug.
Special instructions
Caution is advised to prescribe a drug with xanthine oxidase inhibitors, diuretics, zidovudine, in acute renal failure.
Inosine pranobex, like other antiviral agents, is most effective for acute viral infections if treatment is started at an early stage of the disease (preferably from the first day).
Since inosine is excreted in the form of uric acid, with prolonged use it is recommended to periodically monitor the concentration of uric acid in blood serum and urine. Patients with a significantly increased concentration of uric acid in the body can simultaneously take drugs that lower its concentration.
It is necessary to control the concentration of uric acid in the blood serum when using inosine pranobex simultaneously with drugs that increase the concentration of uric acid or drugs that impair renal function.
In elderly patients, an increase in the concentration of uric acid in blood serum and urine is more likely than in middle-aged patients.
Use with caution in patients with acute liver failure, since the drug is metabolized in the liver. In patients with hepatic insufficiency, serum and urine levels of uric acid should be monitored every 2 weeks. Monitoring the activity of liver enzymes is recommended every 4 weeks with long courses of treatment with the drug.
Influence on the ability to drive vehicles and control mechanisms
When used in patients involved in driving vehicles and other potentially dangerous activities, the possibility of dizziness and drowsiness should be considered.
Composition
Per 100 ml of syrup:
Active ingredient:
Inosine pranobex 5.00 g
Excipients:
Sucrose 5,00.00 g
Methyl parahydroxybenzoate 0.18 g
Propyl parahydroxybenzoate 0.02 g
Citrus flavor 0.50 g
Purified water to 100 ml
Dosage and administration of
Take orally.
Adults: 500 mg to 4 g / day.
Children 3 to 12 years of age: 50 mg / kg / day.
In both adults and children, in severe infectious diseases, the dose can be individually increased to 100 mg / kg body weight / day, divided into 4-6 doses.
The maximum daily dose for adults is 3-4 g, for children – 50 mg / kg.
Frequency of administration, course of treatment, frequency of repeated courses depends on the indications, course of the disease, treatment regimen.
Side effects
From the nervous system: often – headache, dizziness, fatigue, feeling ill often – nervousness, drowsiness, insomnia.
From the gastrointestinal tract: often – decreased appetite, nausea, vomiting, epigastric pain infrequently – diarrhea, constipation.
From the hepatobiliary system: often – increased activity of liver enzymes, alkaline phosphatase.
From the skin and subcutaneous fat: often – itching, rash.
From the kidneys and urinary tract: infrequently – polyuria.
Allergic reactions: infrequently – maculopapular rash, urticaria, angioedema.
From the musculoskeletal system: often – joint pain, exacerbation of gout.
Other: often – increased blood urea nitrogen concentration.
Drug Interactions
Immunosuppressants weaken the immunostimulating effect of inosine pranobex.
When used simultaneously with xanthine oxidase inhibitors (allopurinol), or loop diuretics (furosemide, torasemide, ethacrine acid), an increase in the concentration of uric acid in blood serum is possible.
Joint use with zidovudine leads to an increase in the concentration of zidovudine in blood plasma and lengthens its T1 / 2. With this combination, a dose adjustment of zidovudine may be required.
When combined, it enhances the effects of interferon-alpha, antiviral acyclovir and zidovudine.
Overdose
In case of overdose gastric lavage is indicated, symptomatic therapy.
Storage conditions
Store in a dark place at a temperature of 15 to 25 ° C.
Keep out of the reach of children.
The Expiration of
is 3 years.
Expiration after first opening of the vial – 3 months.
Do not use after the expiry date stated on the package.
pharmacy terms and conditions without a prescription
Dosage form
syrup