Description
Latin name
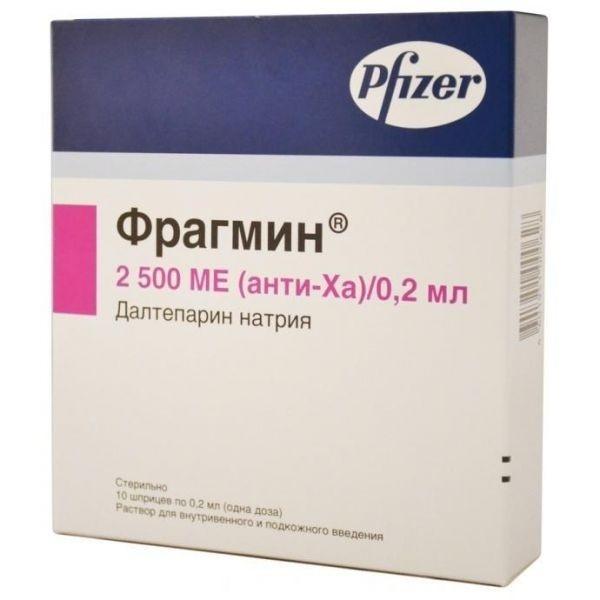
Fragmin
Release form
Solution for iv and sc administration.
Packaging
10 syringes of 0.2 ml.
Indications
– An acute period of traumatic brain injury mainly with stem lesions (including with impaired consciousness coma).
– Ischemic and hemorrhagic type of cerebral circulation disorders (in the acute and recovery period).
– Degenerative and involutional psycho-organic syndromes and the consequences of cerebrovascular insufficiency, such as primary and secondary disorders of mnemonic functions, characterized by impaired memory, confusion, disorientation, decreased motivation, initiative and ability to concentrate.
– Changes in the emotional and behavioral sphere: emotional instability, irritability, decreased interest.
– Senile pseudomelancholy.
Contraindications
– bleeding (clinically significant, for example, from the gastrointestinal tract on the background of gastric ulcer and duodenal ulcer, intracranial bleeding)
– a history of or suspected immune thrombocytopenia (caused by heparin)
– recent injuries or surgical interventions on the central nervous system, organs of vision, hearing
– septic endocarditis
– pronounced disorders of the blood coagulation system increased srdlp sodium or other low molecular weight heparins and / or heparin.
Due to the high risk of high dose Fragmin bleeding (used for example for the treatment of acute deep vein thrombosis, pulmonary embolism, unstable angina pectoris and myocardial infarction without Q wave on ECG), patients who are planning to undergo spinal or epidural anesthesia, or others procedures accompanied by lumbar puncture.
Use during pregnancy and lactation
When used in pregnant women, there was no adverse effect on the course of pregnancy, as well as on the health of the fetus and newborn. When using Fragmin during pregnancy, the risk of adverse effects on the fetus is assessed as low. However, since the possibility of adverse effects cannot be completely ruled out, Fragmin can be prescribed only for strict indications, when the intended benefit for the mother outweighs the potential risk.
If it is necessary to use Fragmin during pregnancy, it is necessary to monitor the anticoagulant activity of the drug.
In experimental studies, no teratogenic or fetotoxic effect of the drug was detected.
Not established whether dalteparin sodium excreted in breast milk.
Composition
0.2 ml solution for iv and s / c administration contains: dalteparin sodium 2500 IU anti-Ha.
Excipients: sodium chloride, sodium hydroxide or hydrochloric acid, water d / and.
Dosage and administration of
Fragmin cannot be given in oil!
For the treatment of acute deep vein thrombosis and pulmonary thromboembolism, Fragmin is administered subcutaneously 1-2 times / day. In this case, you can immediately begin therapy with indirect anticoagulants. Such combination therapy should be continued until the prothrombin index reaches a therapeutic level (usually not earlier than after 5 days). Treatment of patients on an outpatient basis can be carried out in doses recommended for therapy in a hospital. With the introduction of 1 time / day, the dose is 200 IU / kg of body weight. A single dose should not exceed 18,000 IU. With the introduction of 2 times / day, a single dose is 100 IU / kg. Monitoring of anticoagulant activity of the drug may not be carried out, but it should be borne in mind that this may be required in the treatment of certain groups of patients. The recommended maximum plasma concentration of the drug should be 0.5-1 IU anti-Xa / ml.
To prevent blood coagulation in the extracorporeal circulation system during hemodialysis or hemofiltration, Fragmin is administered iv.
Patients with chronic renal failure or patients without a history of bleeding in the history usually require a slight correction of the dosing regimen, so there is no need for frequent monitoring of anti-Xa levels. With the introduction of the recommended doses during hemodialysis, an anti-Xa activity level of 0.5-1 IU / ml is usually achieved. With a duration of hemodialysis or hemofiltration of not more than 4 hours, the drug is administered iv in a jet of 30-40 IU / kg body weight, followed by iv drip at a rate of 10-15 IU / kg / h, or once in a dose of 5000 IU. With a duration of hemodialysis or hemofiltration of more than 4 hours, an intravenous injection of the drug is carried out at a dose of 30-40 IU / kg, followed by 10-15 IU / kg / h.
When using Fragmin in patients with acute renal failure or in patients with a high risk of bleeding, the drug is administered iv in a stream of 5-10 IU / kg, followed by iv drip at a rate of 4-5 IU / kg / h. When conducting emergency hemodialysis, more careful monitoring of the level of anti-Xa activity is required, since the range of therapeutic doses for such patients is much narrower. The level of anti-XA activity should be in the range of 0.2-0.4 IU / ml.
For the prevention of thrombosis during surgical interventions, Fragmin is administered sc. Monitoring of anticoagulant activity is generally not required. When using the drug in recommended doses, the maximum plasma concentrations are from 0.1 to 0.4 IU anti-Xa / ml.
When performing an operation in general surgical practice in patients at risk of developing thromboembolic complications, the drug is administered sc at a dose of 2500 IU 2 hours before surgery, then after surgery at 2500 IU / day (every morning) for the entire period the patient is on bed rest (usually 5-7 days). Patients with additional risk factors for thromboembolic complications (including patients with malignant tumors) Fragmin should be used throughout the period while the patient is on bed rest. At the same time, at the start of therapy the day before the operation, Fragmin is administered sc in a dose of 5000 IU on the evening before the operation, then after5000 IU operations every evening. At the beginning of therapy, s / c 2500 IU is administered on the day of the operation 2 hours before the operation and 2500 IU after 8-12 hours, but not earlier than 4 hours after the end of the operation, then 5000 IU every morning from the next day.
When performing orthopedic operations (for example, when hip replacement), Fragmin should be administered within 5 weeks after surgery, choosing one of the alternative dosage regimens. At the beginning of therapy on the evening before the operation, a single dose for sc administration is 5000 IU, and the drug is administered on the evening before the operation and then every evening after the operation. When therapy is started on the day of surgery, Fragmin is administered sc in a dose of 2500 IU 2 hours before the operation and 2500 IU after 8-12 hours, but not earlier than 4 hours after the end of the operation, then 5000 IU every morning from the next day.
When starting therapy after surgery, the drug is administered sc at a dose of 2500 IU 4-8 hours after surgery, then from the next day at 5000 IU / day.
In case of unstable angina pectoris or myocardial infarction without an abnormal Q wave, the recommended maximum plasma concentration of the drug should be 0.5-1 IU anti-Xa / ml (at the same time, acetylsalicylic acid therapy at a dose of 75 to 325 mg / day is advisable). Fragmin is administered sc in 120 IU / kg body weight every 12 hours. The maximum dose should not exceed 10,000 IU / 12 hours. Therapy should be continued until the clinical condition becomes stable (usually at least 6 days) or longer. (at the discretion of the doctor). Then it is recommended to switch to long-term therapy with Fragmin at a constant dose up to revascularization (percutaneous interventions or coronary artery bypass grafting). The total duration of therapy should not exceed 45 days. The dose of Fragmin is selected taking into account the gender and body weight of the patient. Women with a body weight of less than 80 kg and men with a body weight of less than 70 kg should be given a sachet of 5000 IU every 12 hours. Women with a body weight of 80 kg or more and men with a body weight of 70 kg and more should be administered 7500 IU every 12 hours
Side effects of
From the hemopoietic system and blood coagulation system: rarely, reversible non-immune thrombocytopenia, bleeding (when used in high doses) in some cases – immune thrombocytopenia (with or without thrombotic complications) development of spinal or epidural hematoma.
From the digestive system: in some cases, a transient increase in the activity of hepatic transaminases.
Local reactions: hematoma at the injection site, soreness rarely – skin necrosis.
Other: in some cases – anaphylactic reactions.
Drug Interaction
When used with hemostatic drugs such as NSAIDs, thrombolytic agents, other anticoagulants, as well as inhibitors of platelet function, possibly enhancing the antagonizing effect of Fragmin.
When combined with Fragmin with antihistamines, cardiac glycosides, tetracyclines, ascorbic acid, Fragmin may be weakened.
Pharmaceutical interaction. Fragmin is compatible with isotonic sodium chloride solution (9 mg / ml), isotonic glucose solution (50 mg / ml).
Overdose
Treatment: the anticoagulant effect of dalteparin sodium can be eliminated by the administration of protamine sulfate (a means of emergency therapy). 1 mg of protamine partially inhibits 100 IU of dalteparin sodium (and although complete neutralization of the induced increase in clotting time is observed, but 25 to 50% of the anti-Xa activity of dalteparin sodium still persists).
Storage conditions
Keep out of the reach of children at a temperature not exceeding 30 ° C.
Expiration
Expiration is 3 years.
Deystvuyuschee substances
Dalteparyn sodium
pharmacy leave with prescriptions