Description
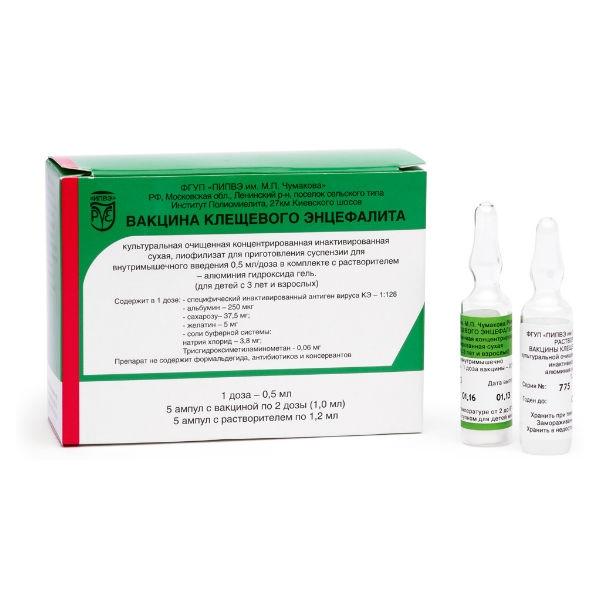
Release form
lyophilized purified concentrated suspension of tick-borne encephalitis virus formalin inactivated.
Packing
1 amp / 1 dose / 0.5 ml No. 5
Pharmacological action of
The vaccine stimulates the production of cellular and humoral immunity to tick-borne encephalitis virus. After two injections of the drug (vaccination course), neutralizing antibodies are detected in no less than 90% of vaccinees.
Indications
Specific prevention of tick-borne encephalitis for children from 3 years of age and adults, immunization of donors in order to obtain specific immunoglobulin.
The contingents to be vaccinated:
The population living in areas enzootic for tick-borne encephalitis, as well as people who have arrived in these territories, performing the following tasks: Agricultural, irrigation and drainage, construction, excavation and transfer of the pound, procurement, fishing, geological, exploration, survey , deratization and pest control.
For logging, clearing and improvement of forests, areas of recreation and recreation.
Persons working with live cultures of tick-borne encephalitis.
Persons visiting endemic areas for tick-borne encephalitis for recreation, tourism, work in summer cottages and gardens.
Contraindications
Acute infectious and non-infectious diseases – vaccinations are carried out no earlier than 1 month after recovery.
Chronic diseases in the acute stage.
Severe allergic reactions in the history of food (especially chicken egg protein), drugs, bronchial asthma, systemic diseases of the connective tissue.
Severe reaction (fever above 40 ° C, edema, hyperemia more than 8 cm in diameter at the injection site) or a complication of the previous dose of the vaccine.
Pregnancy.
When vaccinating donors, the contraindications listed above and the contraindications related to donor selection should be considered.
In each case of a disease not included in this list of contraindications, vaccination is carried out with the permission of the doctor, based on the health status of the vaccinee and the risk of contracting tick-borne encephalitis. In order to identify contraindications, the doctor (paramedic) conducts a survey and examination of the vaccinee with mandatory thermometry on the day of vaccination.
Vaccination against tick-borne encephalitis is carried out no earlier than 1 month after vaccination against another infectious disease. It is allowed to vaccinate against tick-borne encephalitis at the same time (on the same day) with other vaccinations with inactivated vaccines (except for rabies) of the National calendar of preventive vaccinations and the calendar of preventive vaccinations according to epidemic indications.
Special instructions
After administration of the vaccine, local and general reactions may develop in some cases. Local reactions are expressed in redness, swelling, soreness at the injection site, the development of infiltrate. Perhaps a slight increase in regional lymph nodes. The duration of local reactions does not exceed 3 days. General reactions can develop in the first two days after vaccination and are expressed in fever, headache, malaise, their duration does not exceed 48 hours. The frequency of reactions with temperatures above 37.5 ° C should not exceed 7%.
In extremely rare cases, vaccinations can be accompanied by the development of immediate allergic reactions, in connection with which the vaccinees must be under medical supervision for 30 minutes after vaccination. Vaccination sites should be provided with means of antishock and antiallergic therapy.
Composition
One vaccination dose (0.5 ml) of the preparation contains: specific tick-borne encephalitis virus antigen – active component human albumin donor – 250 + 50 Ñg (stabilizer) sucrose – 37.5 + 0.5 mg (stabilizer) gelatin – 5 + 0.5 mg (former) bovine serum albumin – not more than 0.5 Ñg protamine – sulfate – not more than 5 Ñg. The vaccine does not contain formaldehyde, antibiotics, or preservatives.
Dosage and administration of
1. Preventive vaccination.
The vaccination course consists of two intramuscular injections of 1 dose (0.5 ml) with an interval of 1-7 months. The vaccination course (two vaccinations) can be carried out throughout the year, including in the summer period (epidemic season), but no later than 2 weeks before visiting the focus of tick-borne encephalitis.
The most optimal is the interval between the first and second vaccinations of 5-7 months (autumn – spring). Revaccination is carried out once in a dose of 0.5 ml 1 year after completion of the vaccination course. Subsequent long-term revaccinations are carried out once every three years.
Vaccination is carried out with strict adherence to aseptic and antiseptic rules. The vaccine is dissolved in the supplied solvent at the rate of 0.5 ml per dose. The vial with the solvent is vigorously shaken, the necks of the vials are treated with alcohol, opened, collect the solvent into the syringe and place it in an ampoule with a dry vaccine. The contents of the ampoule with the vaccine are vigorously mixed for 3 minutes until the vaccine is completely dissolved, filling it several times into the syringe without foaming.
The vaccine should form a homogeneous suspension within 3 minutes when a solvent for the vaccine is added to the ampoule (0.5 ml per 1 dose and 1.0 ml per 2 doses). Before each injection, the contents of the ampoule are mixed, since upon sedimentation, the suspension is separated into a colorless transparent supernatant and a loose white precipitate, the vaccination is carried out immediately after the inoculation dose into the syringe. The vaccine dissolved in the ampoule cannot be stored.
The drug is not suitable in ampoules with impaired integrity, labeling, if foreign inclusions are detected, with a change in physical properties (severe deformation of the tablet the porous mass of white becomes translucent and melted in shape, color changes, the presence of large, non-breaking conglomerates in the solvent after shaking), with an expired Expiration, in violation of the temperature regime of storage or transportation.
The drug is administered intramuscularly in the region of the deltoid muscle of the shoulder
. The vaccinations performed are recorded in the established accounting forms with the name of the drug, the date of vaccination, dose, batch number, and reaction to the vaccine.
2. Vaccination of donors.
Vaccination course – two injections of 0.5 ml with an interval of 5-7 months or three injections in doses of 0.5 ml for the first and 1.0 ml for the second and third with an interval of 3-5 weeks between vaccinations. The first scheme provides the best immunization effect. Revaccination – a dose of 0.5 ml after 6-12 months. The route of administration is similar to that of prophylactic vaccination. The first blood sampling from donors is carried out 14-30 days after the course of vaccination.
Storage conditions
The drug is stored and transported in accordance with SP 3.3.2. 1248-03 at a temperature of 2 to 8 ° C. Do not freeze. Transportation is allowed at a temperature of from 9 to 25 ° C for 2 days. Over long distances – only by air.
Shelf life
3 years.
active substance
vaccine for the prevention of tick-borne encephalitis
lekarstvennaja form
Solution for
Prescription
For adults prescribed by a doctor, Children prescribed by a doctor, Children over 3 years old
FSUE Institute Chumakov, Russia