Description
Latin name
Azoximera bromide ( Ñ zoximeri bromidum)
Pharmacological action
Pharmacotherapeutic group:
immunomodulating agent.
ATX Code: [L0Z]
Pharmacological properties of
Pharmacodynamics
Azoximer bromide has a complex effect: immunomodulating, detoxifying, antioxidant, moderate anti-inflammatory.
The basis of the mechanism of immunomodulating action of Azoximer bromide is a direct effect on phagocytic cells and natural killers, as well as stimulation of antibody formation and synthesis of interferon-alpha and interferon-gamma.
Detoxification and antioxidant properties of azoximer bromide are largely determined by the structure and high molecular nature of the drug.
Azoximer bromide increases the body’s resistance to localized and generalized infections of bacterial, fungal and viral etiology. Restores immunity in secondary immunodeficiency conditions caused by various infections, injuries, complications after surgery.
A characteristic feature of azoximer bromide in topical (sublingual) use is the ability to activate factors of the body’s early defense against infection: the drug stimulates the bactericidal properties of neutrophils, macrophages, enhances their ability to absorb bacteria, increases the bactericidal properties of saliva and the secretion of the upper respiratory tract.
With oral administration of Azoximer, bromide also activates lymphoid cells in the lymph nodes of the intestine.
Azoximera bromide blocks soluble toxic substances and microparticles, has the ability to remove toxins, salts of heavy metals from the body, inhibits lipid peroxidation, both by intercepting free radicals and by eliminating catalytically active Fe2 + ions. Azoximer bromide reduces the inflammatory response by normalizing the synthesis of pro- and anti-inflammatory cytokines.
Azoximera bromide is well tolerated, does not have mitogenic, polyclonal activity, antigenic properties, does not have allergenic, mutagenic, embryotoxic, teratogenic and carcinogenic effects.
Azoximer bromide is odorless and tasteless, has no local irritant effect when applied to the mucous membranes of the nose and oropharynx.
Pharmacokinetics of
Azoximer bromide after oral administration is rapidly absorbed from the digestive tract, the bioavailability of the drug when administered orally is more than 70%. The maximum plasma concentration is reached 3 hours after ingestion. Pharmacokinetics Azoximer bromide is linear (plasma concentration is proportional to the dose taken).
Azoximer bromide is a hydrophilic compound. The apparent volume of distribution is approximately 0.5 l / kg, which suggests that the drug is distributed mainly in the intercellular fluid. The half-absorption period is 35 minutes, the half-life is 18 hours.
Azoximer bromide is rapidly distributed throughout all organs and tissues of the body, penetrates the blood-brain and blood-brain barrier. No cumulative effect. In the body of Azoximer, bromide undergoes biodegradation to low molecular weight oligomers, excreted mainly by the kidneys, with feces – not more than 3%.
Indications
Used in adults and children from 3 years of age for the treatment and prevention of acute and chronic respiratory diseases in the acute stage and remission.
For treatment (in complex therapy):
of acute and exacerbation of chronic recurrent infectious and inflammatory diseases of the oropharynx, paranasal sinuses, upper and lower respiratory tract, inner and middle ear
allergic diseases (including hay fever, bronchial asthma) complicated by recurrent bacterial, fungal and viral infections
For prophylaxis (monotherapy):
recurrent herpes simplex infection of the nasal and exacerbations of chronic foci of infections of the oropharynx, paranasal sinuses, upper respiratory tract, inner and middle ear
secondary immunodeficiency states resulting from a hundred rhenium or exposure to adverse factors.
Contraindications
Increased individual sensitivity
pregnancy, lactation
children under 3 years of age
acute renal failure
rare hereditary intolerance to lactose, lactase deficiency, glucose galactosorption syndrome.
With caution
If you have the diseases indicated in this section, consult your doctor before taking the drug:
– chronic renal failure (apply no more than 2 times a week).
Use during pregnancy and lactation
The use of the drug Polyoxidonium ® is contraindicated for pregnant women and women during breastfeeding (there is no clinical experience with the use).
During the experimental use of the drug Polyoxidonium ® in animals, no embryotoxic and teratogenic effects or effects on the development of the fetus were revealed.
Before using Polyoxidonium ®, if you are pregnant, or suspect that you might be pregnant, or plan to become pregnant, you should consult your doctor.
Consult a physician before breastfeeding before using Polyoxidonium ®.
Special instructions
If an allergic reaction develops, stop using Polyoxidonium ® and consult a doctor.
If you need to stop taking the drug Polyoxidonium ® cancellation can be done immediately, without gradually reducing the dose.
If you miss the introduction of the next dose of the drug, its subsequent use should be carried out in the usual manner, as indicated in this manual or recommended by a doctor. The patient should not administer a double dose in order to compensate for missed doses.
Do not use the drug if there are visual signs of its unsuitability (packaging defect, discoloration of the tablet).
Impact on the ability to drive vehicles and other mechanisms
The use of Polyoxidonium ® does not affect the ability to perform potentially hazardous activities that require an increased concentration of attention and speed of psychomotor reactions (including driving, working with moving mechanisms).
Composition
Active ingredient:
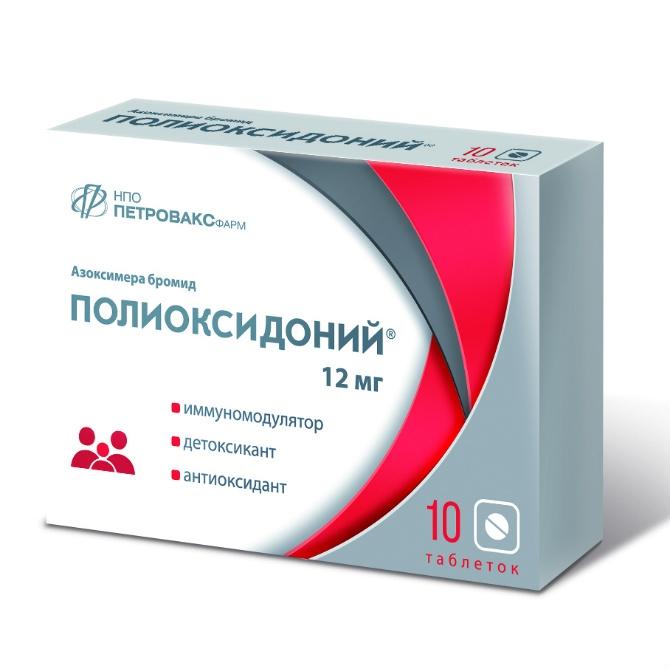
Azoximera bromide – 12 mg
Excipients:
mannitol – 3.6 mg,
povidone K 17 – 2.4 mg,
lactose monohydrate – 185.0 mg,
krach – 45 mg 0 mg,
stearic acid – 2.0 mg.
Dosage and administration of
Use the drug only according to the indications the method of use and in the doses indicated in the instructions.
If, after treatment, improvement does not occur or the symptoms worsen, or new symptoms appear, consult a doctor.
Orally and sublingually 20-30 minutes before meals daily 2 times a day: for children over 10 years and adults – 1 tablet, for children from 3 to 10 years -? tablets (6 mg).
If necessary, repeated courses of therapy are possible after 3-4 months. With a repeated prescription of the drug, its effectiveness does not decrease.
Recommended treatment regimens
Sublingual
For adults:
influenza and acute respiratory infections – 1 tablet 2 times a day for 7 days
inflammatory processes of the oropharynx – 1 tablet 2 times a day for 10 days
exacerbations of chronic diseases of the upper respiratory tract, paranasal sinuses, chronic otitis media – 1 tablet 2 once a day for 10 days
allergic diseases (including hay fever, bronchial asthma), complicated by recurrent bacterial, fungal and viral infections – 1 tablet 2 times a day for 10 days.
For treatment of children from 3 to 10 years:
of influenza and acute respiratory infections – by? tablets 2 times a day for 7 days
inflammatory processes of the oropharynx – by? tablets 2 times a day for 7 days
allergic diseases (including hay fever, bronchial asthma), complicated by recurrent bacterial, fungal and viral infection – by? tablets 2 times a day for 7 days.
For treatment of children over 10 years old:
of influenza and acute respiratory infections – 1 tablet 2 times a day 7 days
of the inflammatory processes of the oropharynx – 1 tablet 2 times a day for 7 days
exacerbations of chronic diseases of the upper respiratory tract, paranasal paranasal sinuses, chronic otitis media – 1 tablet 2 times a day for 7 days
allergic diseases (including hay fever, bronchial asthma), complicated by recurrent bacterial, fungal and viral infection – 1 tablet 2 times a day 7 days.
For prevention for adults:
influenza and acute respiratory infections in the pre-epidemic period – 1 tablet per day for 10 days
recurrent herpetic infection of the nasal and labial areas – 1 tablet 2 times a day 10 days
exacerbations of chronic foci of infections of the oropharynx, paranasal sinuses, paranasal sinuses , inner and middle ear – 1 tablet once a day for 10 days
secondary immunodeficiencies resulting from aging or exposure to adverse factors – 1 tablet 1 time per day for 10 days.
For prophylaxis for children from 3 to 10 years:
influenza and acute respiratory infections in the pre-epidemic period – by? tablets per day for 7 days
recurrent herpetic infection of the nasal and labial areas – by? tablets 2 times a day for 7 days
exacerbations of chronic foci of infections of the oropharynx, paranasal sinuses, upper respiratory tract, inner and middle ear – by? tablets once a day for 10 days.
For prevention for children over 10 years old:
of influenza and acute respiratory infections in the pre-epidemic period – 1 tablet per day for 7 days
of recurrent herpetic infection of the nasal and labial areas – 1 tablet 2 times a day 7 days
of exacerbations of chronic foci of infection oropharynx, paranasal sinuses, upper respiratory tract, inner and middle ear 1 tablet once a day for 10 days.
Oral
For treatment of adults:
diseases of the upper and lower respiratory tract – 1 tablet 2 times 10 days.
For treatment of children over 10 years of age:
diseases of the upper and lower respiratory tract – 1 tablet 2 times 10 days
Side effects
No side effects reported.
If you notice any side effects not listed in the instructions, notify your doctor.
Drug interaction
Azoximer bromide does not inhibit the isoenzymes CYP1A2, CYP2C9, CYP2C19, CYP2D6, cytochrome P-450, therefore the drug is compatible with antibiotics, antiviral, antifungal and antihistamines, glucocorticosteroids and glucocorticosteroids.
If you are taking the above or other medications (including over-the-counter), consult your doctor before taking Polyoxidonium.
Overdose
Overdose has not been reported.
Storage Conditions
At 2 to 25 ° C.
Keep out of the reach and sight of children.
Shelf life
2 years. Do not use the drug after the expiration date indicated on the package.
Active ingredient
Azoxymer bromide
dosage form
tablets
Possible product names
POLYOXIDONIUM 0.012 N10 TABLE
Polyoxidonium 12mg Tab. X10 (R)
Polyoxidonium 12mg No. 10 tab
POLIOXIDONIUM 12MG. No. 10 TAB.
Polyoxidonium tab 12mg N10
NPO Petrovaks Pharm, Russia