Description
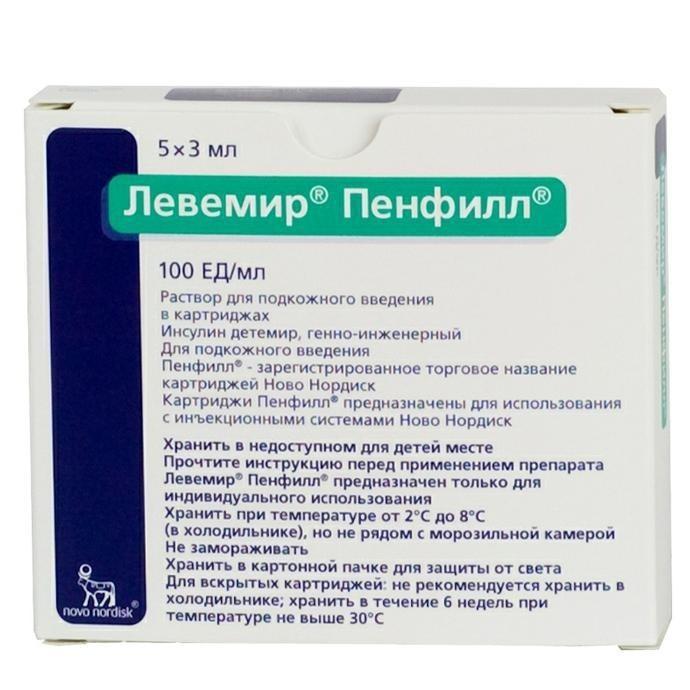
Release form
Solution for subcutaneous injection
Packaging
5 cartridges (3 ml) per pack.
Pharmacological action
Levemir Penfill – a hypoglycemic agent, an analogue of human long-acting insulin. The drug Levemir Penfill is produced by the recombinant DNA biotechnology method using the Saccharomyces cerevisiae strain. It is a soluble basal analogue of human insulin prolonged action with a flat profile of action. The action profile of the drug Levemir Penfill is significantly less variable compared to isofan-insulin and insulin glargine. The prolonged action of the drug Levemir Penfill is due to the pronounced self-association of detemir insulin molecules at the injection site and the binding of the drug molecules to albumin by means of a compound with a side fatty acid chain. Compared with isofan-insulin, detemir insulin is delivered to peripheral target tissues more slowly. These combined delayed distribution mechanisms provide a more reproducible absorption and action profile of Levemir Penfill compared to isofan-insulin. It interacts with a specific receptor on the outer cytoplasmic membrane of cells and forms an insulin-receptor complex that stimulates intracellular processes, including the synthesis of a number of key enzymes (hexokinase, pyruvate kinase, glycogen synthetase, etc.). The decrease in blood glucose is caused by an increase in its intracellular transport, increased tissue uptake, stimulation of lipogenesis, glycogenogenesis, a decrease in the rate of glucose production by the liver, etc. For doses of 0.2 – 0.4 U / kg 50%, the maximum effect of the drug occurs in the range of 3 -4 hours to 14 hours after administration. The duration of action is up to 24 hours, depending on the dose, which makes it possible to administer once or twice daily. After subcutaneous administration, a pharmacodynamic response was proportional to the dose administered (maximum effect, duration of action, general effect). Long-term studies have demonstrated low rates of diurnal fluctuations in plasma glucose concentrations in patients treated with Levemir Penfill, as opposed to isofan-insulin.
Indications
Diabetes mellitus.
Contraindications
Increased individual sensitivity to insulin detemir or any of the components of the drug. It is not recommended to use the drug Levemir Penfill in children under 6 years of age, because clinical trials in children under 6 years of age have not been conducted.
Pregnancy and lactation
Clinical experience with Levemir Penfill during pregnancy and breastfeeding is limited. A study of reproductive function in animals did not reveal differences between insulin detemir and human insulin in terms of embryotoxicity and teratogenicity. Generally, careful monitoring of pregnant women with diabetes during the entire period of pregnancy, as well as when planning pregnancy, is necessary. The need for insulin in the first trimester of pregnancy usually decreases, then in the second and third trimesters it increases. Shortly after birth, the need for insulin quickly returns to the level that was before pregnancy. In lactating women, insulin dosage and dietary adjustments may be required.
Special instructions
Levemir Penfill is a soluble basal insulin analogue with a flat and predictable activity profile with a prolonged effect.
Unlike other insulins, intensive therapy with Levemir Penfill does not increase body weight. The lower risk of nocturnal hypoglycemia compared to other insulins allows for more intensive dose selection in order to achieve the target blood glucose. Levemir Penfill provides better glycemic control (based on fasting plasma glucose measurements) compared with isofan-insulin. An insufficient dose of the drug or discontinuation of treatment, especially with type 1 diabetes, may lead to the development of hyperglycemia or diabetic ketoacidosis. As a rule, the first symptoms of hyperglycemia appear gradually, over several hours or days. These symptoms include thirst, rapid urination, nausea, vomiting, drowsiness, redness and dryness of the skin, dry mouth, loss of appetite, smell of acetone in exhaled air. In type 1 diabetes mellitus, without appropriate treatment, hyperglycemia leads to the development of diabetic ketoacidosis and can lead to death. Hypoglycemia can develop if the dose of insulin is too high in relation to the need for insulin, skipping meals or unplanned intense physical exertion. After compensating for carbohydrate metabolism, for example, with intensified insulin therapy, patients may experience typical symptoms of precursors of hypoglycemia, what patients should be informed about. The usual warning signs may disappear with a long course of diabetes. Concomitant diseases, especially infectious and accompanied by fever, usually increase the body’s need for insulin. The transfer of the patient to a new type or insulin preparation of another manufacturer must occur under strict medical supervision. If you change the concentration, manufacturer, type, species (animal, human, analogues of human insulin) and / or its production method (genetically engineered or insulin of animal origin), dose adjustment may be required. Patients on treatment with Levemir Penfill may need a dose change compared to previously used insulin. The need for dose adjustment may arise after the introduction of the first dose or within the first few weeks or months. As with other insulin treatments, reactions may develop at the injection site, which is manifested by pain, itching, hives, swelling, and inflammation. Changing the injection site in the same anatomical region can reduce symptoms or prevent the development of a reaction. Reactions usually disappear within a few days to several weeks. In rare cases, reactions at the injection sites require discontinuation of treatment. Levemir Penfill should not be administered intravenously, as this can lead to severe hypoglycemia. Intramuscular absorption is faster and to a greater extent compared with subcutaneous administration. If Levemir Penfill is mixed with other insulin preparations, the action profile of one or both components will change. Mixing Levemir Penfill with a fast-acting insulin analogue, such as insulin aspart, leads to an action profile with a reduced and delayed maximum effect compared to their separate administration. Levemir Penfill is not intended for use in insulin pumps.
Influence on the ability to drive a car and work with mechanisms
The ability of patients to concentrate and reaction speed may be impaired during hypoglycemia and hyperglycemia, which can be dangerous in situations where these abilities are especially necessary (for example, when driving or working machines and mechanisms). Patients should be advised to take measures to prevent the development of hypoglycemia and hyperglycemia when driving a car and working with mechanisms. This is especially important for patients with no or diminished symptoms of precursors of developing hypoglycemia or suffering from frequent episodes of hypoglycemia. In these cases, the appropriateness of driving or similar work should be considered.
Composition of
in 1 ml contains: active substance: insulin detemir – 100 PIECES (one cartridge (3 ml) – 300 PIECES)
excipients: glycerol, phenol, metacresol, zinc acetate, sodium hydrogen phosphate dihydrate, sodium chloride, hydrochloric acid or sodium hydroxide, water for injection. One unit of insulin detemir contains 0.142 mg of salt-free insulin detemir. One unit of insulin detemir (ED) corresponds to one unit of human insulin (ME).
Dosage and Administration
Levemir Penfill is intended for subcutaneous administration. The dose and frequency of administration of the drug Levemir Penfill is determined individually in each case. Treatment with Levemir Penfill in combination with oral hypoglycemic drugs, it is recommended to start once a day at a dose of 10 PIECES or 0.1-0.2 PIECES / kg. The dose of Levemir Penfill should be selected individually based on plasma glucose values. If Levemir Penfill is used as part of a basic bolus regimen, it should be prescribed 1 or 2 times a day based on the patient’s needs. Patients who require the use of the drug twice a day to optimally control their glycemia levels can enter the evening dose either during dinner, or before bedtime, or 12 hours after the morning dose. Levemir Penfill is injected subcutaneously in the thigh, anterior abdominal wall or shoulder. The injection sites should be changed even when introduced into the same area.
dose adjustment As with other insulins, in elderly patients and patients with renal or hepatic insufficiency, blood glucose concentration should be closely monitored and the dose of detemir individually adjusted for insulin. Dose adjustment may also be necessary when enhancing the patient s physical activity, changing his normal diet, or with a concomitant illness.
Transfer from other insulin preparations
Transfer from medium-acting insulins and prolonged insulin to Levemir Penfill may require dose and time adjustment. As with other insulin preparations, careful monitoring of blood glucose concentrations during transfer and in the first weeks of a new drug is recommended. Maybe, correction of concomitant hypoglycemic therapy will be required (dose and time of administration of short-acting insulin preparations or dose of oral hypoglycemic drugs).
Side effects
Adverse reactions observed in patients using Levemir Penfill are mainly dose-dependent and develop due to the pharmacological effect of insulin. Hypoglycemia is usually the most common side effect. Hypoglycemia develops if a too high dose of the drug is administered relative to the body’s need for insulin. From clinical studies it is known that severe hypoglycemia requiring third-party intervention develops in approximately 6% of patients receiving Levemir Penfill. Reactions at the injection sites can be observed more often with Levemir Penfill treatment than with the introduction of human insulin. These reactions include redness, inflammation, bruising, swelling, and itching at the injection site. Most reactions at the injection sites are minor and temporary in nature, i.e. disappear with continued treatment for a few days to several weeks. The proportion of patients receiving treatment and who are expected to develop side effects is estimated as 12%. The incidence of side effects, which are generally estimated to be related to Levemir Penfill during clinical trials, is presented below.
Metabolic and nutritional disorders: frequent – Hypoglycemia. Symptoms of hypoglycemia usually develop suddenly. These include cold sweat, pallor of the skin, fatigue, nervousness or tremor, anxiety, unusual tiredness or weakness, disorientation, decreased concentration, drowsiness, severe hunger, blurred vision, headache, nausea, palpitations. Severe hypoglycemia can lead to loss of consciousness and / or convulsions, temporary or irreversible impairment of brain function up to a fatal outcome.
General disorders and reactions at the injection site: frequent – redness, swelling and itching at the injection site. These reactions are usually temporary and disappear with continued treatment.
Rare – Lipodystrophy. It can develop at the injection site as a result of non-compliance with the rule of changing the injection site within the same area.
Edema can occur at the initial stage of insulin therapy. These symptoms are usually temporary.
Immune system disorders: rare – Allergic reactions, urticaria, skin rash. Such symptoms may develop due to generalized hypersensitivity. Other signs of generalized hypersensitivity may include itching, sweating, gastrointestinal upset, angioedema, difficulty breathing, palpitations, and low blood pressure. Generalized hypersensitivity reactions (anaphylactic reactions) are potentially life-threatening.
Visual disturbances: rare – refractive errors, diabetic retinopathy.
Nervous system disorders: very rare – peripheral neuropathy.
Drug Interactions
There are a number of drugs that affect the need for insulin. The hypoglycemic effect of insulin is enhanced by oral hypoglycemic drugs, monoamine oxidase inhibitors, angiotensin converting enzyme inhibitors, carbonic anhydrase inhibitors, non-selective beta-blockers, bromocriptine, sulfonamides, anabolic steroids, tetracyclines, clofibrate, ketoconazole, mebendazole, pyridoxine, theophylline, cyclophosphamide, phenfluramine, lithium preparations, preparations containing ethane. The hypoglycemic effect of insulin is weakened by oral contraceptives, glucocorticosteroids, iodine-containing thyroid hormones, somatropin, thiazide diuretics, heparin, tricyclic antidepressants, sympathomimetics, danazole, clonidine, blockers of slow calcium channels, morphinin dyne, diacidine, diacidine, diacidine, diacidine, diacidine, diacidin both weakening and enhancing the action of the drug. Octreotide / lanreotide can both increase and decrease the body’s need for insulin. Beta-blockers can mask the symptoms of hypoglycemia and delay recovery after hypoglycemia. Alcohol can enhance and prolong the hypoglycemic effect of insulin. Some drugs, for example, containing thiol or sulfite groups, when added to the drug Levemir Penfill, can cause the destruction of insulin detemir. Levemir Penfill should not be added to infusion solutions.
Overdose
No specific dose required for insulin overdose has been established, however, hypoglycaemia may develop gradually if the dose is too high for a particular patient.
Treatment: mild hypoglycemia can be eliminated by the patient taking glucose, sugar or carbohydrate-rich foods. Therefore, patients with diabetes mellitus are advised to carry sugar, sweets, cookies or sweet fruit juice all the time.
In the case of severe hypoglycemia, when the patient is unconscious, 0.5 to 1 mg of glucagon should be given intramuscularly or subcutaneously (may be administered by a trained person), or intravenous dextrose (glucose) solution (only by a healthcare professional). Dextrose should also be administered intravenously if the patient does not regain consciousness 10-15 minutes after glucagon administration. After regaining consciousness, the patient is advised to eat carbohydrate-rich foods to prevent the recurrence of hypoglycemia.
Storage conditions
Store at 2 ° C to 8 ° C (in the refrigerator), but not near the freezer. Do not freeze.
Keep in a cardboard box for protection against light, out of the reach of children.
For open cartridges: it is not recommended to refrigerate. Store for 6 weeks at a temperature not exceeding 30 ° C.
Shelf life
30 months.
Active ingredient
Insulin detemir
Terms and conditions otpuska IZ pharmacy prescription
Novo Nordisk, Denmark