Description
Briefly about the product
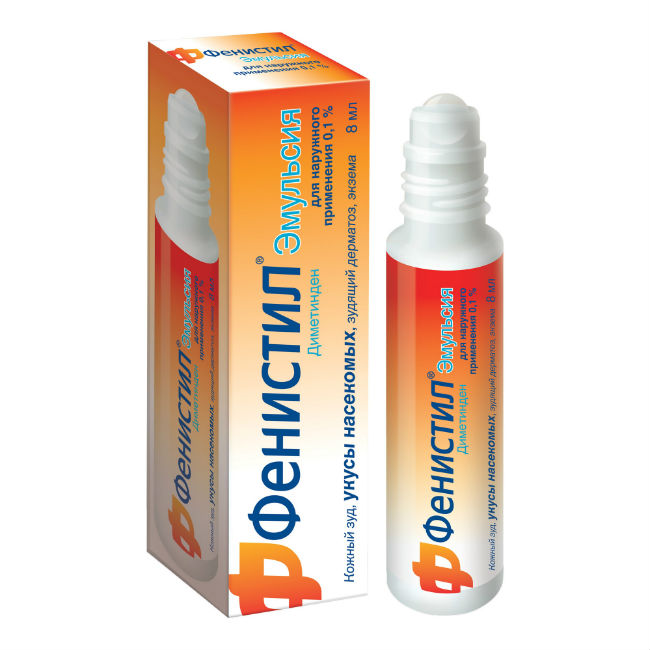
Fenistil Emulsion with a ball applicator reduces itching and irritation with insect bites and skin-allergic reactions * after several minutes.
The emulsion base of the preparation provides cooling, softening and moisturizing effects. *
The emulsion in the format of a convenient and compact vial with a ball applicator is easy to apply. Suitable for adults and children from 1 month. *
* Instructions for medical use, RU No LP-000794 dated 03/10/2011
Description
Emulsion for external use, whitish, homogeneous, semi-liquid, with a slight smell of benzyl alcohol.
Release form
Emulsion.
Pharmacological action
Histamine H1 receptor blocker. It has anti-allergic and antipruritic effect.
Reduces the increased capillary permeability associated with allergic reactions. When applied to the skin, itching and irritation caused by skin-allergic reactions are reduced.
The drug also has a pronounced local anesthetic effect.
Blocks the action of kinins, has a weak anticholinergic effect.
For external use, due to the gel base, action with a slight cooling effect begins in a few minutes and reaches a maximum in 1-4 hours.
Indications
Itchy skin of various origins (except associated with cholestasis) with: – itchy dermatoses
– eczema
– urticaria
– insect bites.
Sunburn, domestic and industrial burns (mild).
Contraindications
– angle-closure glaucoma
– prostatic hyperplasia
– children under 1 month of age (especially premature)
– hypersensitivity to dimethindene and other components that make up the drug.
Fenistil ® should be prescribed with caution in the first trimester of pregnancy and during lactation (breastfeeding).
Contraindicated in children up to 1 month (especially in premature infants).
In infants and young children, the drug should not be used on large areas of the skin, especially in the presence of inflammation or bleeding.
Use during pregnancy and lactation
The use of Fenistil ® in the first trimester of pregnancy is possible only after consulting a doctor.
In the second and third trimesters of pregnancy and during lactation, the gel should not be used on large areas of the skin, especially in the presence of inflammation and bleeding.
Nursing mothers should not apply the drug to the nipples of the mammary glands.
Dosage and administration
Applied externally.
The drug should be applied to affected areas of the skin 2-4 times / day.
In cases of severe itching or widespread skin lesions, the simultaneous use of oral forms is recommended.
Side effects
Determination of the frequency of side effects: very often ( 1/10), often ( 1/100 and <1/10), infrequently ( 1/1000 and <1/100), rarely ( 1 / 10,000 and <1/1000), very rarely (<1/10 000), including individual messages and reactions with an unknown frequency (cannot be estimated based on available data). From the skin and subcutaneous tissue: infrequently – dry skin, a burning sensation of the skin. Allergic reactions: very rare (post-registration data) – allergic dermatitis, including skin rash, itching. If any of the above side effects get worse, or any other side effects appear, the patient should inform your doctor. Drug Interaction The drug interaction of Fenistil ® has not been described. overdose Symptoms: Symptoms specific to histamine H1 receptor blockers may occur during accidental ingestion of a large amount of the drug – CNS depression and drowsiness (mainly in adults), CNS stimulation and m-cholin block, .h. excitation, ataxia, tachycardia, hallucinations, tonic or clonic convulsions, mydriasis, dry mouth, blood tides to the face, urinary retention, fever. This may be followed by a decrease in blood pressure. Treatment: The specific antidote is unknown. Activated charcoal should be assigned, salt laxative to undertake activities to maintain the function of the cardiovascular and respiratory systems. For the treatment of arterial hypotension, vasoconstrictor agents can be used. In case of an accidental overdose, the patient should consult a doctor. Storage conditions The drug should be stored out of the reach of children, gel at a temperature not exceeding 25 ° C. Expiration 3 years. Deystvuyuschee substances Dymetynden dosage form dosage form emulsion for external application GSK Consumer Helsker SA, Switzerland