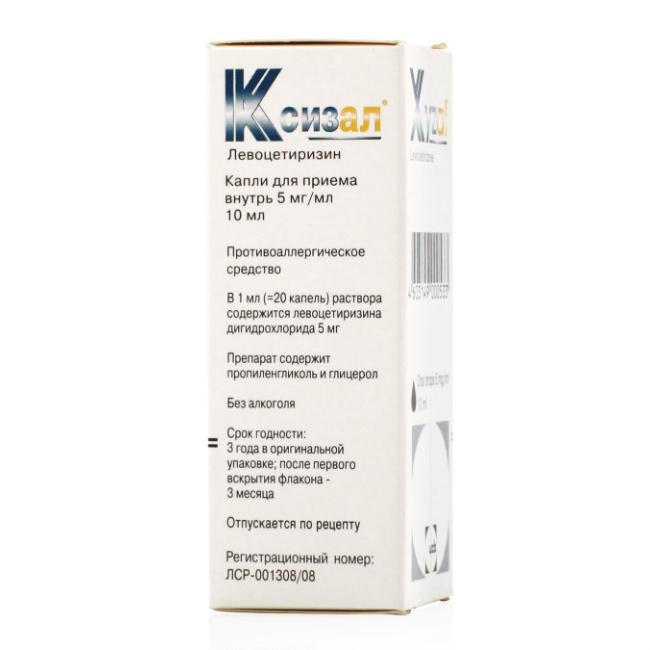
Description
Latin name
Xyzal
Release form
Drops for oral administration.
Packing
10 ml.
Indications
Symptomatic treatment of allergic diseases and conditions:
perennial (persistent) and seasonal (intermittent) allergic rhinitis and allergic conjunctivitis (pruritus, sneezing, rhinorrhea, lacrimation, conjunctival hypertrophy) including chronic idiopathic urticaria)
Quincke’s edema
other allergic dermatoses accompanied by itching and rashes.
Pregnancy and lactation
Experimental animal studies have not revealed any direct or indirect adverse effects of levocetirizine on the developing fetus, as well as the development in the postnatal period, the course of pregnancy and childbirth also did not change. Adequate and strictly controlled clinical studies on the safety of the drug during pregnancy have not been conducted, therefore, levocetirizine should not be prescribed during pregnancy. Levocetirizine is excreted in breast milk, therefore, its use is possible only if the intended benefit to the mother outweighs the potential risk to the baby.
Composition
1 ml contains:
Active ingredient: levocetirizine dihydrochloride 5 mg.
Excipients:
sodium acetate, acetic acid,
propylene glycol,
glycerol 85%,
methyl parahydroxybenzoate,
propyl parahydroxybenzoate,
sodium saccharin,
.
Dosage and administration of
Xizal is taken orally, without chewing, with food or on an empty stomach, washed down with a small amount of water. For adults and children over 6 years old – in a daily dose of 5 mg. The duration of treatment depends on the disease: with hay fever, an average of 1-6 weeks is prescribed for chronic diseases (year-round rhinitis, atopic dermatitis), the duration of treatment can increase up to 18 months.
Drug Interactions
There has been no study of the interaction of levocetirizine with other drugs. When studying the drug-drug interaction of cetirizine racemate with pseudoephedrine, cimetidine, ketoconazole, erythromycin, azithromycin, glipizide and diazepam, no clinically significant adverse interactions were detected. With simultaneous administration with theophylline (400 mg / day), the total clearance of cetirizine is reduced by 16% (the kinetics of theophylline does not change). In some cases, with the simultaneous use of levocetirizine with alcohol or drugs that depress the central nervous system, it is possible to increase their effect on the central nervous system, although it has not been proven that the cetirizine racemate potentiates the effect of alcohol.
Overdose
Symptoms: drowsiness (in adults), agitation and anxiety, replaced by drowsiness (in children).
Treatment: Immediately after ingestion it is necessary to wash the stomach or cause artificial vomiting. The appointment of activated charcoal, symptomatic and supportive therapy is recommended. There is no specific antidote. Hemodialysis is not effective.
Storage Conditions
In a dark place at a temperature not exceeding 30 ° C.
Active ingredient
Levocetirizine
prescription
Prescription
Dosage form
drops for ingestion
Prescription
For children prescribed by the doctor, Children over 2 years old, Adults prescribed by a doctor
YUSB Pharma SA, Belgium