Description
Brief about
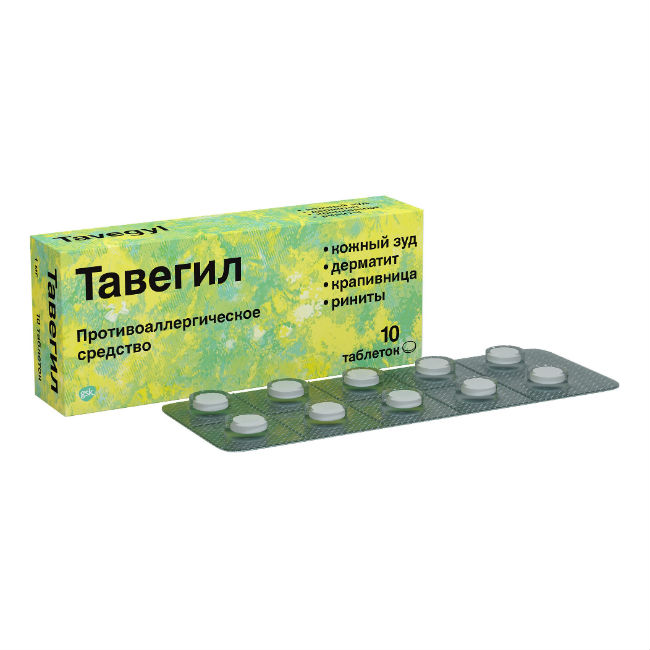
Tavegil product – antihistamine first generation.
Active ingredient – clemastine hydrofumarate *.
Available in ampoule and tablet format (10 and 20 pieces).
* Instructions for medical use, RU Ñ N008878 / 02 dated 02.06.2010
Description
White or almost white tablets, round, flat, with a beveled edge, on one side pills of risk and engraving FROM.
Latin name
TAVEGYL
Release form
Tablets.
Pharmacological action
Histamine H1 receptor blocker, ethanolamine derivative.
It has a strong antihistamine and antipruritic effect with a rapid onset of action and a duration of up to 12 hours, prevents the development of histamine-induced vasodilation and smooth muscle contraction.
With anti-allergic effect, reduces the permeability of blood vessels, capillaries, inhibits exudation and the formation of edema, reduces itching, has an m-anticholinergic effect.
Indications
Pollinosis (hay fever, incl. allergic rhinoconjunctivitis)
urticaria of various origin
itching, itchy dermatoses
acute and chronic eczema, contact dermatitis
drug allergy
insect bites.
Contraindications
Diseases of the lower respiratory tract (including bronchial asthma)
simultaneous use of MAO inhibitors
children under 6 years old
pregnancy
lactation (breastfeeding)
component of the drug sensitivity.
Tavegil ® should be used with caution in patients with stenotic gastric ulcer, with pyloroduodenal obstruction, with obstruction of the bladder neck, as well as prostatic hypertrophy, accompanied by urinary retention, with increased intraocular pressure, hyperthyroidism, diseases of the cardiovascular system (including with hypertension).
Use during pregnancy and lactation
The use of the drug during pregnancy and during breastfeeding is contraindicated.
Special instructions
To prevent distortion of the results of skin scarification tests for allergens, the drug must be canceled 72 hours before an allergological test.
The tablets contain lactose, so the drug is not recommended for patients suffering from rare congenital diseases, associated with galactose intolerance, severe lactase deficiency and malabsorption of glucose-galactose.
Pediatric use of
Tavegil ® in tablet form is contraindicated in children under 6 years of age. For the treatment of children from the age of 1 year, Tavegil ® can be used in the form of a solution for intravenous and intramuscular administration.
Influence on the ability to drive vehicles and control mechanisms
Clemastine has a slight sedative effect (mild to moderate in intensity), therefore, patients taking Tavegil ® are advised to refrain from driving vehicles, working with machinery, as well as from other types activities requiring increased attention and speed of psychomotor reactions.
Composition
1 tablet contains:
Active substances:
clemastine 1 mg, which is equivalent to 1.34 mg of clemastine hydrofumarate.
Excipients:
lactose monohydrate,
povidone (polyvinylpyrrolidone),
corn starch,
magnesium stearate,
talc.
Dosage and administration of
Inside adults and children over 12 years of age are prescribed 1 tab. (1 mg) morning and evening. In cases difficult to treat, the daily dose can be up to 6 tablets. (6 mg).
Children aged 6-12 years are prescribed 1 / 2-1 tablets. before breakfast and at night.
Tablets should be taken before meals with water.
Side effects
Determination of frequency of adverse reactions: very often ( 1/100, <1/10) infrequently ( 1/1000, <1/100) rarely ( 1/10 000, <1/1000) very rarely (<1 / 10,000). From the nervous system: often – fatigue, drowsiness, sedation, weakness, fatigue, tiredness, violation of coordination of movements infrequently – dizziness is rare – headache, tremor, stimulating action. From the digestive system: rarely – dyspepsia, nausea, vomiting, gastralgia very rarely – constipation, dry mouth in some cases – decreased appetite, diarrhea. From the respiratory system: rarely – thickening of the bronchial secretion and difficulty in sputum separation, feeling of pressure in the chest and difficulty breathing, nasal congestion. From the cardiovascular system: rarely – lowering blood pressure (more often in elderly patients), extrasystole very rarely – palpitations. From the organs of the senses: rarely – a violation of the clarity of visual perception, diplopia, acute labyrinthitis, tinnitus. On the part of the urinary system: very rarely – increased urination, difficulty urinating. From the hematopoietic system: rarely hemolytic anemia, thrombocytopenia, agranulocytosis. Dermatological reactions: rarely skin rash, photosensitization. Allergic reactions: rarely anaphylactic shock. Drug Interaction Tavegil ® potentiates the action of CNS depressants (hypnotics, sedatives, tranquilizers), m-cholin blockers, and ethanol. Incompatible with the simultaneous administration of MAO inhibitors. Overdose Symptoms: an overdose of antihistamines can lead to both a depressing and stimulating effect on the central nervous system. CNS stimulation is more common in children. Manifestations of an anticholinergic action may also develop: dry mouth, fixed dilated pupils, flushing of the upper body, gastrointestinal tract disorders (nausea, epigastric pain, vomiting). Treatment: if the patient does not vomit spontaneously, then it should be induced artificially (only if the patient s consciousness is preserved). If 3 hours or more have elapsed since the drug was taken, gastric lavage can be performed using isotonic sodium chloride solution. You can also prescribe a saline laxative. Symptomatic therapy is also indicated. Storage conditions Do not store above 30 ° C. Keep out of the reach and sight of children. Expiration 5 years. pharmacy leave terms pharmacy leave pharmacies without leave Novartis Farma Stein AG, Switzerland