Description
Latin name
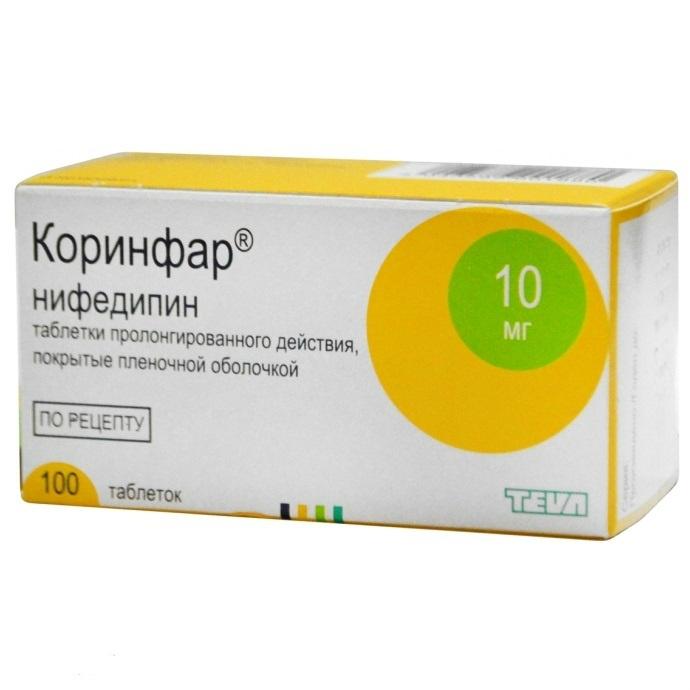
Corinfar
Release form
Coated tablets.
Packaging
In a dark glass vial, 100 coated tablets. In a pack of cardboard 1 bottle.
Indications
– Stable angina pectoris.
– Arterial hypertension.
– Angiospastic angina pectoris (Prinzmetal angina, variant angina pectoris).
Contraindications
– Cardiogenic shock.
– Severe stenosis of the aortic orifice.
– Unstable angina pectoris.
– Acute myocardial infarction.
– Pregnancy.
– Lactation (breastfeeding).
– Hypersensitivity to nifedipine.
Use during pregnancy and lactation
Corinfar is contraindicated in pregnancy.
If you need to use Corinfar during lactation, you should decide on the termination of breastfeeding.
Composition
1 coated tablet contains: nifedipine 10 mg.
Dosage and administration
Set individually depending on the severity of the disease.
In the treatment of stable and angiospastic angina pectoris and arterial hypertension, the drug is prescribed 10 mg (1 tab.) 2-3 times / day. With insufficient severity of the clinical effect, a gradual increase in dose to 20-40 mg (2-4 tablets) is possible 2 times / day.
The maximum daily dose is 80 mg. The interval between doses of two single doses of 20 mg (2 tablets) should not be less than 4 hours. With a double administration of the drug per day, tablets should be taken after about 12 hours (morning and evening). The drug should be taken after a meal, without chewing and drinking plenty of fluids.
Side effects
From the cardiovascular system: tachycardia, palpitations, arrhythmias, peripheral edema (ankles, feet, legs), manifestations of excessive vasodilation (asymptomatic decrease in blood pressure, development or worsening of heart failure, flushing of the face, flushing of the egg skin, feeling hot), marked decrease in blood pressure (rarely), syncope. In some patients, especially at the beginning of treatment or when the dose is increased, angina attacks may occur and in rare cases, myocardial infarction develops, which requires drug withdrawal.
From the side of the central nervous system: headache, dizziness, general weakness, fatigue, drowsiness. With prolonged use of the drug in high doses – limb paresthesia, tremor, extrapyramidal (parkinsonian) disorders (ataxia, masky face, shuffling gait, tremor of the hands and fingers, difficulty swallowing), depression.
From the digestive system: dyspepsia (nausea, diarrhea or constipation), dry mouth, flatulence, increased appetite. Rarely – gingival hyperplasia, completely disappearing after drug withdrawal. With prolonged use – impaired liver function (intrahepatic cholestasis, increased activity of hepatic transaminases).
From the side of the musculoskeletal system: arthritis, myalgia, swelling of the joints, cramps of the upper and lower extremities.
Allergic reactions: rarely – skin itching, urticaria, exanthema, autoimmune hepatitis, exfoliative dermatitis, photodermatitis, anaphylactic reactions
From the hemopoietic organs: anemia, leukopenia, thrombocytopenia, thrombocytopenic purpurenica.
From the urinary system: an increase in daily urine output, impaired renal function (in patients with renal failure).
Other: rarely – visual impairment (including transient blindness with a maximum concentration of nifedipine in the blood plasma), gynecomastia (in elderly patients, disappearing after cancellation), galactorrhea, hyperglycemia, pulmonary edema, bronchospasm, weight gain.
Drug Interaction
When used with Corinth with beta-blockers, diuretics, and other antihypertensive drugs, there is an increase in the antihypertensive effect of Corinth.
When used with Corinth with nitrates, the anti-anginal effect of Corinth is increased.
With the use of Corinth and tricyclic antidepressants, cimetidine, ranitidine, the effects of Corinth may be enhanced.
With the concomitant use of Corinth with quinidine, a decrease in the concentration of quinidine in the blood plasma was observed in some cases.
With the concomitant use of Corinth with digoxin and theophylline, in some cases a decrease in the concentration of the latter in blood plasma was observed.
overdose
Symptoms: in severe cases – loss of consciousness up to coma, arterial hypotension, tachy / bradycardia, hyperglycemia, metabolic acidosis, hypoxia, cardiogenic shock with pulmonary edema.
Treatment: Abundant gastric lavage, if necessary, in combination with small bowel lavage. The use of laxatives should take into account the decrease in intestinal muscle tone up to the bowel atony observed with the use of calcium channel blockers. Since nifedipine is not excreted in hemodialysis, it is impractical to carry it out. Plasmapheresis is recommended.
At development of a bradycardia it is necessary to appoint atropine and / or beta-sympathomimetics, at the threatening life of the patient of a bradycardia it is necessary to implant the driver of a heart rhythm on time.
In case of arterial hypotension, which is a consequence of cardiogenic shock and expansion of arterial vessels, 1-2 g of calcium gluconate is introduced into / into, in / in a drop – dopamine (up to 25 mcg / kg body weight / min), dobutamine – up to 15 mcg / kg body weight / min), epinephrine (adrenaline) or norepinephrine (norepinephrine). The calcium content of the serum is maintained at a normal or slightly elevated level.
The introduction of fluid or blood into the body is carried out with caution under the control of hemodynamic parameters.
Shelf life
3 years.
Deystvuyushtee substance
Nifedipine
Dosage form
tablets prolong.
Possible product names
CORINFAR 0.01 N100 TABLE P / O
CORINFAR 0.01 N100 TABLE PROLONG P / O
Corinfar 10mg Tab. prolong. valid p / pl / rev X100 B (R)
Corinpar 10mg # 100
Corinpar 10mg # 100 Tab
Teva Pharmaceutical Enterprises Ltd., Israel