Description
Latin name
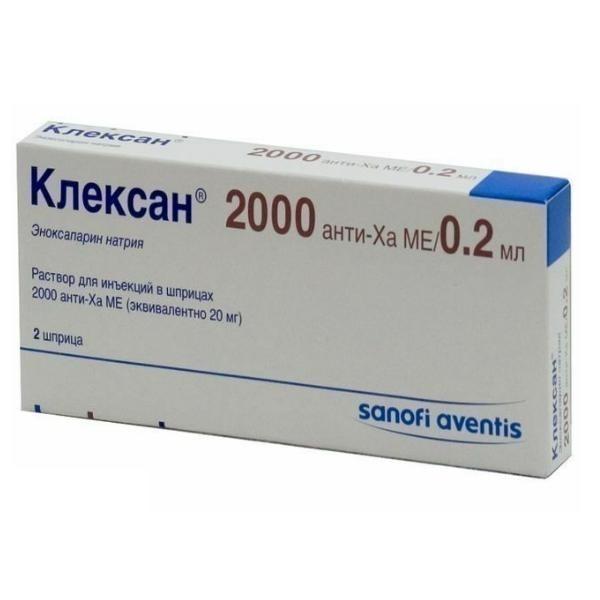
Clexane
Release form
Injection.
Packing
10 pcs
Pharmacological action
Clexane has an antithrombotic effect.
Indications
Prevention of venous thrombosis and embolism, especially during orthopedic and general surgical operations
prevention of venous thrombosis and embolism in patients with acute therapeutic diseases who are in bed rest (chronic heart failure, III, NY class IV) acute respiratory failure acute infection acute rheumatic conditions in combination with one of the risk factors for venous thrombosis
treatment of deep vein thrombosis in combination with or without pulmonary embolism
treatment of unstable angina pectoris and myocardial infarction without Q wave in combination with acetylsalicylic acid in extracorporeal blood flow during hemodialysis.
Contraindications
condition and disease, at which there is a high risk of bleeding (threatening abortion, cerebral aneurysm or stratified aortic aneurysm / with the exception of surgery), hemorrhagic stroke, uncontrolled bleeding, severe enoxaparin- or heparin-induced thrombocytopenia)
age under 18 years (efficacy and safety not established)
hypersensitivity to enoxaparin, heparin and its derivatives, including other low molecular weight heparins
, the use of Clexane is not recommended in pregnant women with artificial heart valves.
Pregnancy and lactation
There is no evidence that enoxaparin sodium crosses the human placenta during the second trimester of pregnancy. There is no information regarding the first and third trimesters of pregnancy. Therefore, Clexane should not be used during pregnancy unless the potential benefit to the mother outweighs the potential risk to the fetus.
Breastfeeding should be discontinued during maternal treatment with Clexane.
Composition
1 syringe contains enoxaparin sodium 2000 anti-XA ME.
Dosage and administration
Clexane is administered subcutaneously in the prone position in the anterolateral and posterolateral region of the abdominal wall at the level of the belt. Prevention of postoperative venous thrombosis and thromboembolism – 20 mg once a day for 7 days (first dose 2 hours before surgery), at very high risk – 40 mg / kg daily, for 10 days (first dose 12 hours before surgery )
Deep vein thrombosis – 1 mg / kg every 12 hours or 1.5 mg / kg once a day for 10 days.
Prevention of coagulation during hemodialysis – 0.5-1 mg / kg in the arterial line at the beginning of hemodialysis, carried out for 4 hours
Treatment of unstable angina and myocardial infarction without Q wave – 1 mg / kg every 12 hours until the condition stabilizes (usually 3-8 days), while taking acetylsalicylic acid.
Side effects
Bleeding
If bleeding develops, the drug must be discontinued, the cause of the bleeding must be established, and appropriate therapy started.
In 0.01-0.1% of cases, hemorrhagic syndrome, including retroperitoneal and intracranial bleeding, is possible. Some of these cases were fatal.
When applying Clexane against spinal / epidural anesthesia and postoperative use of penetrating catheters, cases of spinal cord hematoma are described (in 0.01-0.1% of cases), which leads to neurological disorders of varying severity, including persistent or irreversible paralysis.
Thrombocytopenia
During the first days after initiation of therapy, mild, transient, asymptomatic thrombocytopenia may develop. In less than 0.01% of cases, the development of immune thrombocytopenia in combination with thrombosis, which can sometimes be complicated by heart attack or limb ischemia, is possible.
Local reactions
After sc administration of Clexane, pain at the injection site may be observed, in less than 0.01% of cases, a hematoma at the injection site. In some cases, at the injection site, the formation of solid inflammatory nodules-infiltrates containing the drug is possible, which disappear after a few days and are not grounds for drug withdrawal. In 0.001% of cases, skin necrosis may develop at the injection site, preceded by purpura or erythematic plaques (infiltrated and painful). In these cases, therapy with Clexane should be discontinued.
Other
In 0.01-0.1% of cases, skin or systemic allergic reactions may develop. There have been cases of allergic vasculitis (in less than 0.01% of cases). Some patients may need to stop treatment.
An asymptomatic and reversible increase in the activity of “liver” enzymes was noted.
overdose
Symptoms: accidental overdose with I / O, extracorporeal or p / to administration can lead to hemorrhagic complications. When taken inside, even in large doses, the absorption of the drug is unlikely.
Treatment: Slow in / in administration of protamine sulfate, the dose of which is dependent on the dose of Klexane administered, is shown as a neutralizing agent. It should be borne in mind that 1 mg of protamine counteracts the anticoagulant effect of 1 mg of enoxaparin if Clexane was administered not more than 8 hours before the introduction of protamine. 0.5 mg protamine counteracts the anticoagulant effect of 1 mg Klexane, if it was administered more than 8 hours ago or if a second dose of protamine was required. If more than 12 hours have passed after the administration of Klexan, then the introduction of protamine is not required. However, even with the introduction of protamine sulfate at high doses of anti-XA, Clexan activity is not completely neutralized (by a maximum of 60%).
Storage conditions
In the dark place at a temperature of no higher than 25 ° C.
Expiration
3 years.
Deystvuyuschee substances
noksaparyn sodium
Dosage form
dosage form
injection
Sanofi-Aventis, France