Description
Latin name
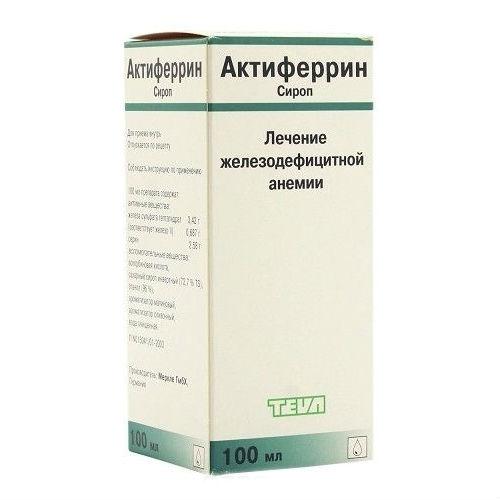
Aktiferrin
Packing
In a bottle of 100 ml.
Pharmacological action
Activerrin syrup – a drug containing iron.
Iron is necessary for the life of the body: it is part of hemoglobin, myoglobin, a number of enzymes reversibly binds oxygen and is involved in a number of redox reactions that stimulate erythropoiesis.
The alpha-amino acid serine, which is part of Actiferrin, promotes more efficient absorption of iron and its entry into the systemic circulation, which leads to a quick restoration of the normal iron content in the body. This provides better tolerance to the drug and reduces the required dose of iron.
When using the Actiferrin drug, iron deficiency in the body is quickly filled up, which leads to a gradual regression of clinical (weakness, fatigue, dizziness, tachycardia, soreness and dry skin) and laboratory symptoms of anemia.
Indications
Iron deficiency anemia of various etiologies.
Latent iron deficiency in the body associated with: Excessive iron loss (bleeding, including uterine constant donation)
Increased need for it (pregnancy, lactation, period of active growth, malnutrition, chronic gastritis with secretory insufficiency, condition after gastrectomy , peptic ulcer of the stomach and duodenum, decreased body resistance in infectious diseases, tumors).
Contraindications
Hypersensitivity to the components of the drug
Iron overload (hemochromatosis, hemosiderosis)
Other types of anemia, not caused by iron deficiency in the body
Aplastic and hemolytic anemia
Siderohrestichnaya anemia, anemia with lead poisoning
Late skin porphyria
Chronic hemolysis.
Precautions: Peptic ulcer and duodenal
Inflammatory bowel disease
Enteritis
diverticulitis
Ulcerative colitis
Crohn
Alcoholism (active or in remission)
Allergic diseases
Asthma
Hepatitis
renal or hepatic insufficiency
Rheumatoid arthritis
blood transfusion Diabetes mellitus.
Use during pregnancy and lactation
With established iron deficiency in the body during pregnancy and lactation (breastfeeding), the use of Actiferrin is considered reasonable and safe.
Composition
100 ml of syrup contains iron sulfate 3.42 g, D, L-serine 2.58 g
excipients: ascorbic acid, invert syrup (72.7% dry syrup), ethanol 96%, raspberry flavor , creamy flavor, purified water.
Dosage and administration of
Actiferrin is taken orally immediately before or during meals. The syrup is taken with a small amount of liquid (fruit tea or water). If your doctor has not prescribed other dosages, you should strictly adhere to the instructions below.
Syrup is the most convenient form for children over 2 years old.
Daily dose: 5 ml per 12 kg of body weight.
Preschool children: the average dose is 5 ml 2-3 times / day.
School children: the average dose is 5 ml 1-2 times / day.
After reaching normal levels of serum iron and hemoglobin, treatment with the drug is continued for at least 8-12 weeks, prescribing a maintenance dose.
Note: to open the bottle, press the cap down and simultaneously turn in the direction of the arrow. After applying the medicine, install the cap and tighten it tightly (prevents access for children).
Side effects of the
From the digestive system: rarely – a feeling of heaviness in the epigastric region, flatulence, constipation or diarrhea, which disappear with a decrease in dose.
Drug Interaction
Specific Antidote – Deferoxamine (desferal). phosphates, oxalates), pancreatin, pancreatolipase, etidrone, caffeine, reduce the absorption of Actiferrin (the interval between the administration of these drugs should be 1-2 hours)
When combined, ascorbic acid increases the absorption of iron
Actiferrin decreases the absorption of fluoroquinolones, penicillamine, tetracyclines (these drugs are recommended for taking 2 hours before or 2 hours after taking iron preparations).
High doses of iron preparations reduce the renal absorption of zinc preparations (the latter is recommended to be taken 2 hours after taking iron preparations).
overdose
Symptoms: at accidental administration of the drug in very high doses, weakness, fatigue, paresthesia, paleness of the skin, decrease in blood pressure, palpitations, acrocyanosis, abdominal pain, diarrhea with vomiting, vomiting, tingling , hyperthermia, lethargy, seizures, symptoms of hyperventilation, coma.
Signs of peripheral vascular collapse appear within 30 min after ingestion of metabolic acidosis, convulsions, fever, leukocytosis, coma – within 12-24 h acute renal and hepatic necrosis – in 2-4 days.
Treatment: before specific therapy – take measures to remove from the stomach not yet absorbed drug (gastric lavage), give milk, raw eggs.
Specific therapy is performed by the administration of deferoxamine (desferal) inside and parenterally. In acute poisoning for the binding of iron, not yet absorbed from the gastrointestinal tract, give inside 5-10 g of diferoxamine by dissolving the content of 10-20 vials in drinking water.
In the development of the phenomena of poisoning, deferoxamine is injected in / m slowly, children – 15 mg / h, adults – 5 mg / kg / h (up to 80 mg / kg / day) with mild poisoning – in / m children 1 g every 4 -6 h, adults – 50 mg / kg (up to 4 g / day).
In severe cases, accompanied by the development of shock, carry out in / in drip administration of the drug at a dose of 1 g and carry out symptomatic therapy.
Hemodialysis is ineffective for iron removal, but can be used to accelerate the removal of iron-deferroxamine complex, and can also be prescribed for oligo- and anuria. Peritoneal dialysis is possible.
Storage conditions
Store in a dry place at a temperature not exceeding 25 ° C.
Expiration
2 Year
Active ingredient
Iron sulfate, Serine
pharmacy prescription
Dosage form
dosage form
oral solution
Teva Pharmaceutical Enterprise Co., Ltd., Israel