Description
Latin name
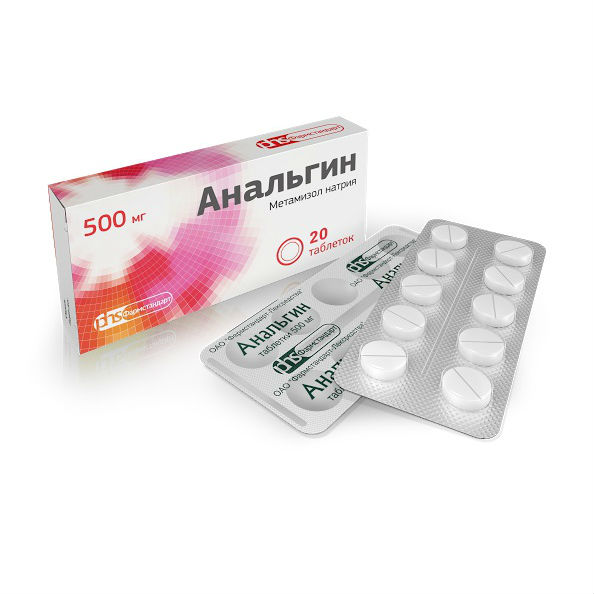
Analgin
Release form
Tablets are white or slightly yellowish in color, with a bitter taste.
packaging 20 pcs
Pharmacological action
Analgin nonselectively blocks cyclooxygenase and reduces the formation of prostaglandins from arachidonic acid, interferes with pain extra- and proprioceptive impulses.
Analgin has a mild anti-inflammatory effect, causing a small effect on water-salt metabolism (sodium and water retention) and the gastrointestinal mucosa.
It has an antispasmodic effect on the smooth muscles of the urinary and biliary tract. The action develops 20-40 minutes after ingestion.
Pharmacokinetics
Well and rapidly absorbed in the digestive tract, which ensures the rapid development of the clinical effect. When taken in therapeutic doses, it passes into breast milk.
Maximum plasma concentration is achieved 1-1.5 hours after ingestion.
In the intestinal wall, it is hydrolyzed to form an active metabolite, 4-methyl-amino-antipyrine, which in turn is metabolized to 4-formyl-amino-antipyrine and other metabolites.
The level of binding of the active metabolite to proteins is 50-60%.
Excretion of metabolites passes through the kidneys. In addition, metabolites are excreted in breast milk.
Indications
Pain (mild to moderate): including headache, migraine, toothache, neuralgia, myalgia, radicular syndrome, algodismenorrhea with renal, hepatic and biliary colic (in combination with antispasmodics),
to reduce pain after surgical and diagnostic procedures,
elevated body temperature with colds and other infectious and inflammatory diseases. The feasibility of using the drug is decided in each case, depending on the severity, nature and tolerance of the fever.
Contraindications
Hypersensitivity to pyrazolone derivatives (propiphenazone, phenazone or phenylbutazone)
bronchial asthma induced by acetylsalicylic acid, salicylates or other non-steroidal anti-inflammatory drugs (NSAIDs, including history), the state after CABG
inhibition of hematopoiesis (agranulocytosis, cytostatic or infectious neutropenia)
expression renal impairment of liver or kidney function
hepatic porphyria
confirmed hyperkalemia, erosive and ulcerative changes in the mucous membrane of the stomach and duodenum, active gastrointestinal bleeding, inflammatory bowel disease, anemia, leukopenia
hereditary hemolytic anemia associated with deficiency of glucose-6-phosphate dehydrogenase.
Use during pregnancy and lactation
Analgin should not be taken during the first and last three months of pregnancy.
From the fourth to the sixth month of pregnancy, Analgin should be taken for strict medical reasons.
After taking Analginum, breast-feeding should be stopped for 48 hours.
Composition
1 tablet contains: active substance: metamizole sodium (analgin) 500 mg
excipients:
sucrose (sugar),
potato starch,
talc,
calcium stearate.
Dosage and administration
Inside, after eating, adults and children over 14 years of age are prescribed 250-500 mg 2-3 times a day, the maximum single dose is 1000 mg, the daily dose is 3000 mg.
Children 8-14 years of age are prescribed 250 mg (1/2 tablet) 2-3 times a day.
Duration of admission without consulting a doctor is not more than 5 days.
Side effects of the
From the urinary system: impaired renal function, oliguria, anuria, proteinuria, very rarely the development of acute interstitial nephritis, staining of urine in red (due to the release of the metabolite – rubazonic acid).
Allergic reactions: urticaria, including on the conjunctiva and mucous membranes of the nasopharynx, Quincke’s edema in rare cases – malignant exudative erythema (Stevens-Johnson syndrome), anaphylactic shock, toxic epidermal necrolysis (Lyell syndrome), only bronchospastic syndrome to bronchospasm).
From the hemopoietic organs: leukopenia, rarely agranulocytosis and thrombocytopenia of immune origin.
Other: possible decrease in blood pressure, heart rhythm disturbance.
Drug Interaction
Concomitant administration of Analgin with other non-narcotic analgesics can lead to mutual enhancement of toxic effects.
Tricyclic antidepressants, contraceptives, and allopurinol interfere with the metabolism of sodium metamizole in the liver and increase its toxicity.
Barbiturates and phenylbutazone weaken the action of Analgin.
Analgin enhances the effects of alcohol-containing beverages.
X-ray contrast drugs, colloidal blood substitutes and penicillin should not be used during metamizole sodium treatment.
Metamizole sodium, by displacing oral hypoglycemic drugs, indirect anticoagulants, glucocorticosteroids and indomethacin, from the protein’s association, increases their activity.
Concomitant administration of Analgin with cyclosporine reduces the latter’s level in the blood.
Thiamazole and sarcolysin increase the risk of leukopenia.
The effect is enhanced by codeine, H2-histamine receptor blockers, propranolol (slows down inactivation).
Sedatives and tranquilizers enhance analgesic analgesic action.
Myelotoxic drugs increase the hematotoxicity of the drug.
Overdose
Symptoms: nausea, vomiting, epigastric pain, vestibular disorders, tinnitus, clonic and tonic convulsions, coma, agranulocytosis, aplastic anemia, hemorrhagic action, hemorrhagic action.
Treatment: gastric lavage, standard detoxifying measures, if necessary – symptomatic therapy.
Storage conditions
In a dark place, out of reach of children, at a temperature not exceeding 25 ° C
Expiration
5 years.
Medicines should not be used after the time stated on the pack.
Deystvuyuschee substances
metamizol sodium
Dispensing conditions from
pharmacies Without a prescription
Dosage form
Dosage form
tablets
Pharmstandard-Leksredstva, Russia