Description
Latin name
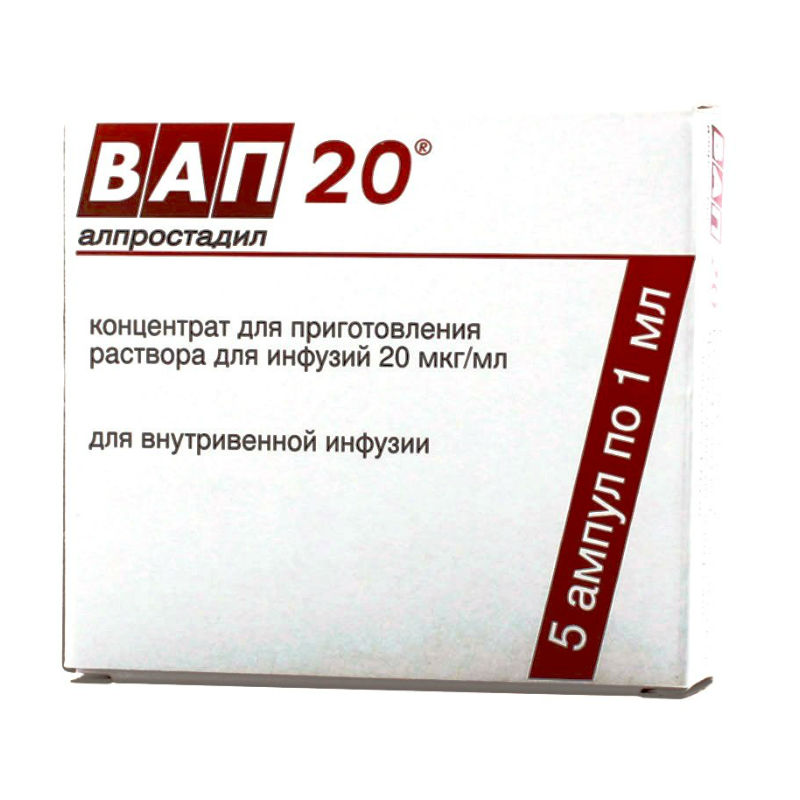
Vap 20
Release form
VAP 20. Concentrate for solution for infusion
Packing
5 ampoules of 1 ml.
Pharmacological action
An analogue of natural PGE1 (prostaglandin E1), has a vasodilator (at the level of arterioles, precapillary sphincters, muscle arteries), antiplatelet and angioprotective effects. It improves microcirculation and peripheral circulation, promotes the opening of collateral vessels. Reduces total peripheral vascular resistance, blood pressure, reflexively increases the heart rate, which leads to an increase in the minute volume of blood.
Improves the rheological properties of blood, increasing the elasticity of red blood cells and reducing platelet adhesion / aggregation. It has a fibrinolytic effect.
Stimulates smooth muscles of the intestines, bladder and uterus, inhibits the secretion of gastric juice
Indications
Chronic obliterating diseases of arteries III and IV stages (according to Fontaine’s classification).
Contraindications
Hypersensitivity to alprostadil or other components of the drug.
Chronic heart failure, severe cardiac arrhythmias, exacerbation of coronary heart disease, myocardial infarction or stroke suffered in the last 6 months.
Pulmonary edema, infiltrative lung disease, chronic obstructive pulmonary disease.
Dysfunction of the liver (increased activity of aspartate aminotransferase, alanine aminotransferase or gamma-glutamyl transferase) and severe liver diseases in the anamnesis, as well as diseases accompanied by an increased risk of bleeding (peptic ulcer, 12 duodenal ulcer, extensive injury).
Concomitant therapy with vasodilator and anticoagulant agents.
Pregnancy and lactation.
General contraindications for infusion therapy, such as decompensated heart failure, pulmonary or cerebral edema, renal functional impairment (oliguria), and hyperhydration.
Children under 18 years old.
Precautions:
Use of VAP20 for arterial hypotension, cardiovascular failure, in patients undergoing hemodialysis (treatment with the drug should be carried out in the post-dialysis period), in patients with diabetes mellitus, especially for diabetic angiopathy.
Use during pregnancy and lactation
Use during pregnancy is contraindicated, breast-feeding should be discontinued.
Composition
Composition 1 ampoule contains:
active substance: alprostadil * 20 μg
excipients: ethanol &
* Alprostadil = PGE1 (prostaglandin E1).
Dosage and Administration
Intravenously administered. The solution should be prepared immediately before administration. 1-2 ampoules (20 μg or 40 μg) are dissolved in 0.9% sodium chloride solution or 5% dextrose solution to a volume of 100-250 ml. The solution, ready for use, is suitable for 24 hours if stored in a refrigerator (2-8 ° C) in a dark place. Do not use a solution prepared more than 24 hours ago.
50-250 ml of the ready-made solution for infusion, the concentration of alprostadil is 40 μg, administered intravenously for 2 hours (333 ng / min), the infusion rate of 0.4-2 ml / min. VAP20 is administered 1 time per day, with severe clinical symptoms of the disease it is possible to administer VAP20 2 times a day. The average duration of treatment is 14 days, with a positive effect, treatment with the drug can be continued for another 7-14 days. The course of treatment should not exceed 4 weeks. In the absence of a positive effect within 2 weeks from the start of treatment, further use of the drug should be discontinued.
In case of impaired renal function (serum creatinine concentration of more than 1.5 mg / dl), intravenous administration begins with 20 mcg. If necessary, after 2-3 days, a single dose is increased to 40-60 mcg. For patients with renal failure and heart failure, the maximum volume of injected fluid is 50-100 ml / day. The course of treatment is 4 weeks.
Side effects of
With the infusion of
From the nervous system and sensory organs: headache, dizziness, paresthesia, convulsive syndrome, increased fatigue, feeling unwell, impaired sensitivity of the skin and mucous membranes rarely – confusion, psychosis.
From the cardiovascular system and blood (hematopoiesis, hemostasis): lowering blood pressure, tachycardia, cardialgia, heart rhythm disturbances, AV block, redness of the skin rarely – the development or deepening of heart failure, leukopenia or leukocytosis.
From the respiratory system: shortness of breath rarely – respiratory distress syndrome, acute pulmonary edema.
From the digestive tract: discomfort in the epigastric region, nausea, vomiting, diarrhea, increased activity of hepatic transaminases rarely – hyperbilirubinemia.
From the genitourinary system: rarely – renal failure, hematuria.
From the musculoskeletal system: arthralgia with prolonged continuous use (more than 4 weeks) – reversible hyperostosis of the bones.
Allergic reactions: skin rashes, urticaria, itching.
Other: increased sweating, hyperthermia, chills, swelling of the extremities, infusion into the vein is rare – an increase in the titer of the C-reactive reaction protein at the injection site – pain, swelling, erythema, impaired sensitivity, phlebitis proximal to the site of IV administration (up to 40% sick).
Extremely rare (up to 1% of cases): shock, acute heart failure, hyperbilirubinemia, bleeding, drowsiness, bradypnea, decreased respiratory function, tachypnea, anuria, impaired renal function, hypoglycemia, ventricular fibrillation, AV block II degree, supraventricular arrhythmia, neck muscle tension, increased irritability, hypothermia, hypercapnia, hyperemia of the skin, hematuria, peritoneal symptoms, tachyphylaxis, hyperkalemia, thrombocytopenia,.
In newborns: apnea (frequency 10 12%), fever (14%), hyperemia of the skin (about 10%, especially with intravenous administration), bradycardia (7%), decreased blood pressure (4%), seizures (4%), tachycardia (3%) about 2% – diarrhea, sepsis about 1% – cardiac arrest, edema, DIC, hypokalemia 1% – cerebral hemorrhage, excessive extension of the neck, hypothermia, nervous agitation, lethargy, congestive heart failure, II degree AV block, supraventricular tachycardia, ventricular fibrillation, bradypnea, stridor, hypercapnia, respiratory depression, tachypnea, regurgitation of gastric contents, hyperbilirubinemia, anemia, bleeding, thrombocytopenia, anuria, hematuria, peritoneal symptoms, hypoglycemia, hyperkalemia with a long course of treatment (for several weeks) hyperostosis in the bones of the lower extremities was reported (see also precautions “).
With intracavernous administration
Local reactions: pain in the penis (37%), excessively long erection (up to 4-6 hours – 4%), hematoma (3%) and ecchymosis (2%) at the injection site (associated with improper technique introduction), the formation of fibrous nodules and Peyronie s disease (3 8%), edema of the penis (1%), rashes on the penis (1%) less than 1% – priapism (0.4%), balanitis, hemorrhage at the injection site inflammation, itching and swelling at the injection site, bleeding from the urethra, penile disorders, including sensation of heat in the penis, numbness, fungal infection, irritation, hypersensitivity, phimosis, rash, erythema, venous discharge, painful erection, impaired ejaculation.
Systemic effects (rare): pain and swelling of the scrotum and testicles, increased urination, urinary incontinence, fluctuations in blood pressure (increase or decrease), tachycardia, supraventricular extrasystole, impaired peripheral circulation, fatigue, headache, hypesthesia, leg swelling, nausea, hyperhidrosis dry mouth, increased serum creatinine, spasms of the calf muscles, weakness in the buttocks, localized pain (in the chest, buttocks, pelvis, legs, genitals, abdomen), mydriasis, flu-like syndrome.
Drug Interaction
Alprostadil may enhance the effect of antihypertensives, vasodilators and anti-anginal agents. Concomitant use of alprostadil in patients receiving blood clotting agents (anticoagulants, antiplatelet agents) may increase the likelihood of bleeding. In combination with cefamandol, cefoperazone, cefotethan and thrombolytics – increases the risk of bleeding. Adrenomimetics – epinephrine, norepinephrine – reduce the vasodilating effect.
It should be borne in mind that drug interactions are possible even if the above agents were used shortly before drug therapy was started.
overdose
An overdose of the drug may be manifested by a decrease in blood pressure (BP) and an increase in heart rate (HR). It is possible to develop vasovagal reactions with pale skin, increased sweating, nausea and vomiting, which may also be accompanied by myocardial ischemia and symptoms of heart failure, as well as pain, swelling and redness of the tissue at the infusion site.
For overdose symptoms, reduce the dose or stop the infusion. With a marked decrease in blood pressure, the patient in the supine position should raise his legs.
Storage conditions
At a temperature of 2 to 8 ° C (in the refrigerator), in a dark place.
Shelf suitability
3 Year
Deystvuyuschee substances
Alprostadil
Dosage form
Dosage form
solution infusion
BEG, Germany