Description
Release form
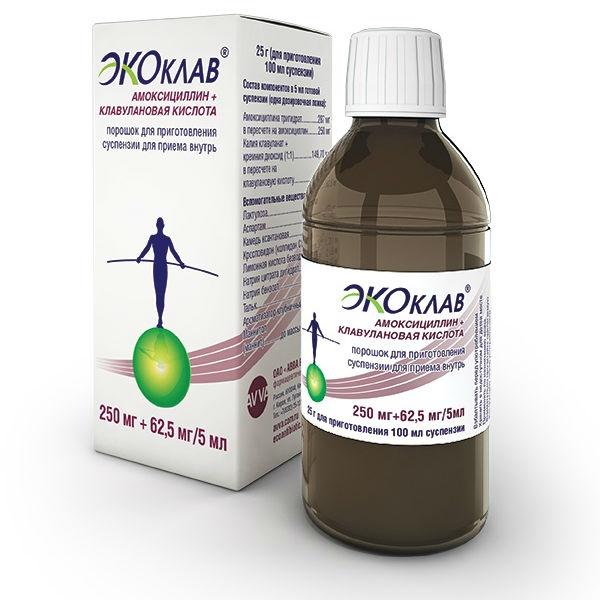
Poro ok for suspension for oral
packaging
Bottle of 25 g
Pharmacological action
Antibacterial.
Indications
Infectious and inflammatory diseases caused by drug-sensitive pathogens:
lower respiratory tract infections (bronchitis, pneumonia)
infections of the ENT organs (sinusitis, tonsillitis, otitis media)
infections of the urinary tract, pyelitis and pyelitis , cystitis, urethritis, bacterial prostatitis, cervicitis, salpingitis, salpingoophoritis, endometritis, bacterial vaginitis, septic abortion, mild chancre, gonorrhea)
infections of the skin and soft tissues (erysipelas, impetigo, secondarily infected dermatoses, abscesses, phlegmon, wound infections)
infections of bones and joints (osteomyelitis).
Contraindications
hypersensitivity (including to cephalosporins and other beta-lactam antibiotics)
infectious mononucleosis
episodes of jaundice or impaired hepatic function as a result of the use of amoxicillin / clavulanic acid in children with less than 12 years of age 40 kg (for this dosage form)
phenylketonuria (contains aspartame) for suspension.
Precautions: severe liver failure, diseases of the gastrointestinal tract (including a history of colitis associated with the use of penicillins), chronic renal failure.
Pregnancy and lactation
The combined preparation of amoxicillin and clavulanic acid during pregnancy is recommended only in cases when the expected benefits of taking it for the mother outweighs the potential risk to the fetus.
The drug can be used during breastfeeding. With the exception of the risk of sensitization associated with the penetration into breast milk of trace amounts of the active ingredients of this drug, no other adverse effects were detected in breast-fed infants.
Composition of
5 ml of the finished suspension (one dosage spoon) contains:
Active substances: amoxicillin trihydrate (in terms of amoxicillin) 250 mg, potassium clavulanate (in terms of clavulanic acid) 62, 5 mg
Excipients: lactulose, aspartame, silicon dioxide, colloidal dioxide (Aerosil), xanthan gum, crospovidone (CL-M collidone), anhydrous citric acid, sodium citrate dihydrate, sodium benzoate, talc, orange flavor, mannitol pf704 (4) manfold4fruf4 (4) Composition
5 ml of the finished suspension (one dosage spoon) contains:
Active substances: amoxicillin trihydrate (in terms of amoxicillin) 250 mg, potassium clavulanate (in terms of clavulanic acid) 62.5 mg
Excipients: lactulose, aspartame, colloidal silica (Aerosil), xanthan gum, crospovidone (Kollidon CL-M), citric acid anhydrous, sodium citrate dihydrate, sodium benzoate, talc, orange flavoring, mannitol (mannitol)
Dosage and administration
The suspension is taken orally. The dosage regimen is set individually depending on the patient s body weight, severity and localization of the infectious process, as well as the sensitivity of the pathogen.
The minimum course of antibiotic therapy is from 5 days. Treatment should not be continued for longer than 14 days without a review of the clinical situation. The duration of treatment for acute uncomplicated otitis media is 5-7 days, in children under 2 years – 7-10 days.
A single dose is determined depending on age and body weight (calculation for amoxicillin): Children up to 3 months – 30 mg / kg / day in 2 doses
Children 3 months and older: – low doses (for the treatment of infections of the skin and soft tissues as well as chronic tonsillitis) – 20 mg / kg / day in 3 doses
– high doses (for the treatment of otitis media, sinusitis, lower respiratory tract infections, urinary tract infections) – 40 mg / kg / day in 3 divided doses.
Children weighing 40 kg or more should be prescribed doses as adults.
Suspension may be used in adults with difficulty swallowing.
Recommended dosing regimen for adults: 20 ml of a suspension at a dosage of 125 mg + 31.25 mg / 5 ml or 10 ml of a suspension at a dosage of 250 mg + 62.5 mg / 5 ml 2-3 times / day.
The maximum daily dose of amoxicillin for adults and children over 12 years old is 6 g, for children under 12 years old – 45 mg / kg body weight.
The maximum daily dose of clavulanic acid for adults and children over 12 years old is 600 mg, for children under 12 years old – 10 mg / kg.
Patients with impaired renal function:
Dose adjustment is based on the maximum recommended dose of amoxicillin and creatinine clearance value.
Kids: Creatinine clearance greater than 30 ml / min dose adjustment is not required
Creatinine clearance 10-30 ml / min – 15 mg / 3.75 mg / kg 2 times / day, maximum dose 500 mg + 125 mg (20 ml suspension in a dosage of 125 mg + 31.25 mg / 5 ml or 10 ml of suspension at a dosage of 250 mg + 62.5 mg / 5 ml) 2 times / day
Creatinine clearance less than 10 ml / min – 15 mg / 3.75 mg / kg 1 time / day, maximum daily dose of 500 mg + 125 mg (20 ml of a suspension at a dosage of 125 mg + 31.25 mg / 5 ml or 10 ml of a suspension at a dosage of 250 mg + 62.5 mg / 5 ml)
Adults: creatinine clearance greater than 30 ml / min dose adjustment not required
creatinine clearance 10- 30 ml / min – 500 mg + 125 mg (20 ml of a suspension at a dosage of 125 mg + 31.25 mg / 5 ml or 10 ml of a suspension at a dosage of 250 mg + 62.5 mg / 5 ml) 2 times / day
Creatinine clearance less than 10 ml / min – 500 mg + 125 mg (20 ml of a suspension in a dosage of 125 mg + 31. 25 mg / 5 ml or 10 ml of suspension at a dosage of 250 mg + 62.5 mg / 5 ml) 1 time / day
Hemodialysis patients: Dose adjustment is based on the maximum recommended dose of amoxicillin
Children: 15 mg / 3.75 mg / kg 1 times / day
One additional dose of 15 mg / 3.75 mg / kg should be taken before a hemodialysis session. To restore the concentration of the active components of the drug in the blood, a second additional dose of 15 mg / 3.75 mg / kg should be taken after a hemodialysis session.
Adults: 500 mg + 125 mg (20 ml of a suspension at a dosage of 125 mg + 31.25 mg / 5 ml or 10 ml of a suspension at a dosage of 250 mg + 62.5 mg / 5 ml) once every 24 hours.
Optional: 1 dose during the dialysis session and another dose at the end of the dialysis session (to compensate for the decrease in serum concentrations of amoxicillin and clavulanic acid)
Method of suspension: The suspension is prepared immediately before use. The powder in the vial is pre-shaken, then, adding a small amount of boiled and cooled to room temperature water, stirred to obtain a uniform suspension, then water is added to the mark on the vial. For accurate dosing of the suspension, a double-sided dosage spoon should be used, which must be rinsed well with water after each use. After dilution, the suspension should be stored for no more than 7 days in the refrigerator, but not frozen.
Side effects
The drug is well tolerated. Side effects rarely occur, they are mostly mild and are transient in nature.
From the digestive system: nausea, vomiting, diarrhea, gastritis, stomatitis, glossitis, cholestatic jaundice, hepatitis, liver failure (more often in the elderly, men, with prolonged therapy), colitis (including pseudomembranous), black hairy tongue, darkening of tooth enamel, increased activity of hepatic transaminases, increased bilirubin content and alkaline phosphatase activity.
From the hemopoietic organs: a reversible increase in prothrombin time and bleeding time, thrombocytopenia, thrombocytosis, eosinophilia, leukopenia, agranulocytosis, hemolytic anemia.
From the central nervous system: dizziness, headache, hyperactivity, anxiety, behavior change, convulsions.
Allergic reactions: urticaria, erythematous rashes, multiforme exudative erythema, anaphylactic shock, angioedema, exfoliative dermatitis, malignant exudative erythema (Stevens-Johnson syndrome), allergic vasculitis, syndrome, similar to acute osteoarthritis
From the kidneys and urinary tract: interstitial nephritis, crystalluria, hematuria.
Other: candidiasis, development of superinfection.
Drug Interaction
It is not recommended to use a combination preparation of amoxicillin and clavulanic acid with probenecid. Probenecid reduces tubular secretion of amoxicillin, so their co-administration can lead to increased and persistent serum concentrations of amoxicillin, whereby the serum concentration of clavulanic acid does not change.
Diuretics, allopurinol, phenylbutazone, non-steroidal anti-inflammatory drugs and other drugs that block tubular secretion, increase the concentration of amoxicillin (clavulanic acid is eliminated mainly by glomerular filtration).
Antacids, glucosamine, laxatives slow down and reduce the absorption of amoxicillin ascorbic acid – increases.
Allopurinol increases the risk of skin rash.
Like other broad-spectrum antibiotics, a combination drug of amoxicillin and clavulanic acid may reduce the effectiveness of oral contraceptives, and patients should be informed.
The literature describes rare cases of increased international normalized ratio (INR) in patients with the combined use of acenocoumarol or warfarin and amoxicillin. When concurrent use of the combination drug amoxicillin and clavulanic acid with indirect anticoagulants, prothrombin time or MNO should be carefully monitored when prescribing or discontinuing the drug.
Overdose
Symptoms: dysfunction of the gastrointestinal tract and water-electrolyte balance.
Treatment: symptomatic. Hemodialysis is effective.
Storage conditions
Store in a dry, dark place at a temperature not exceeding 25 ° C.
Expiration
2 years.
pharmacy leave terms srdl517 pff17p45 df51745f45frd5prd5prd5prd5prd5 srdl5prd5prd5 srdl545df5drd5 srdl5prd5 srdl545df5dff5df5df45fdf5
pharmacies Prescription
Dosage form
Dosage form
suspension for oral administration