Description
Description
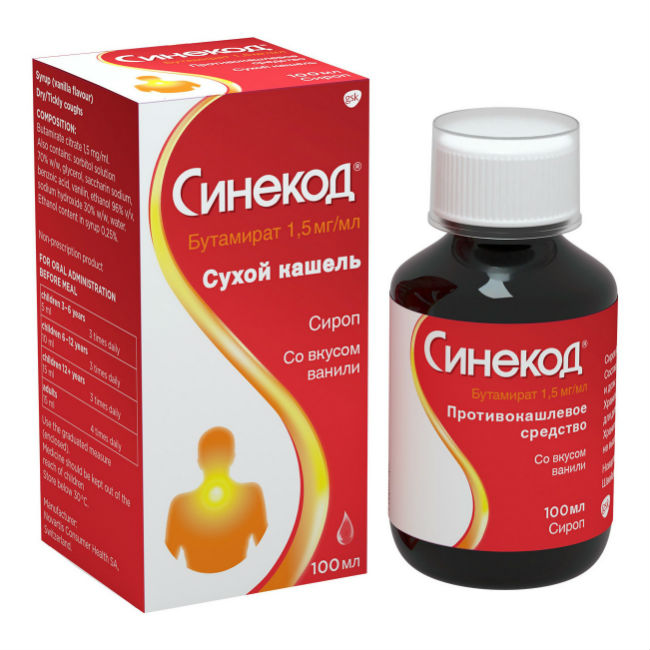
Syrup (vanilla) in the form of a colorless transparent liquid with a vanilla odor.
Release form
100 ml in a dark glass bottle, with a cap made of polyethylene and polypropylene, equipped with a system for unauthorized opening by children, and with a measured cap made of polypropylene.
The bottle, along with instructions for use, is placed in a cardboard box.
Pharmacological action
Antitussive drug of central action, does not apply to opium alkaloids either chemically or pharmacologically.
Does not form addiction or addiction.
suppresses cough, having a direct effect on the cough center. It has a bronchodilating effect.
Helps relieve breathing by improving spirometry (reduces airway resistance) and blood oxygenation.
Indications
Symptomatic treatment of dry cough of various etiologies: suppression of cough in the pre- and postoperative period, during surgical interventions, bronchoscopy, with whooping cough.
Contraindications
Hypersensitivity to the components of the drug
children under 3 years of age (for children under 3 years old you can use Sinecode drops for oral administration for children)
pregnancy (I trimester) and lactation.
Precautions: pregnancy (II and III trimesters).
Use during pregnancy and lactation
There have been no controlled clinical trials in pregnant women.
In this regard, Sinekod ® should not be used in the first trimester of pregnancy. In the II and III trimesters, the use of Sinecode is possible taking into account the benefits to the mother and the potential risk to the fetus.
Given the lack of data on the allocation of butamirate with breast milk, the appointment of the drug during lactation is not recommended.
In animal studies, there was no adverse effect on the fetus.
Composition
Active ingredient:
butamirate citrate – 0.150 (1.5 mg / ml).
Excipients:
sorbitol solution 70% m / m 40.50,
glycerol 29.00.
sodium saccharin 0.06,
benzoic acid 0.115,
vanillin 0.06,
ethanol 96% v / v 0.25,
sodium hydroxide 30% m / m 0.031,
water up to 100 ml.
Side effects
Determination of the frequency of side effects: very often ( 1/10), often ( 1/100 and <1/10), infrequently ( 1/1000 and <1/100), rarely ( 1 / 10,000 and <1/1000), very rarely (<1/10 000), including individual messages. From the nervous system: rarely – drowsiness. From the digestive system: rarely – nausea, diarrhea. Allergic reactions: rarely – urticaria, other manifestations are possible. Drug Interaction The drug interaction of butyramate is not described. Due to the fact that butamirate suppresses the cough effect, simultaneous use of expectorants should be avoided to avoid accumulation of sputum in the respiratory tract with the risk of developing bronchospasm and respiratory tract infection. Overdose Symptoms: drowsiness, nausea, vomiting, diarrhea, dizziness and decrease in blood pressure. Treatment: gastric lavage, activated carbon intake, maintenance of vital functions of the body. There is no special antidote. Storage conditions Store at a temperature not exceeding 30 ° C. Keep out of the reach of children. The Expiration of is 3 years. Deystvuyuschee substances butamirate pharmacy terms and conditions without a prescription Dosage form dosage form oral solution Novartis Farma Stein AG, Switzerland