Description
Latin name
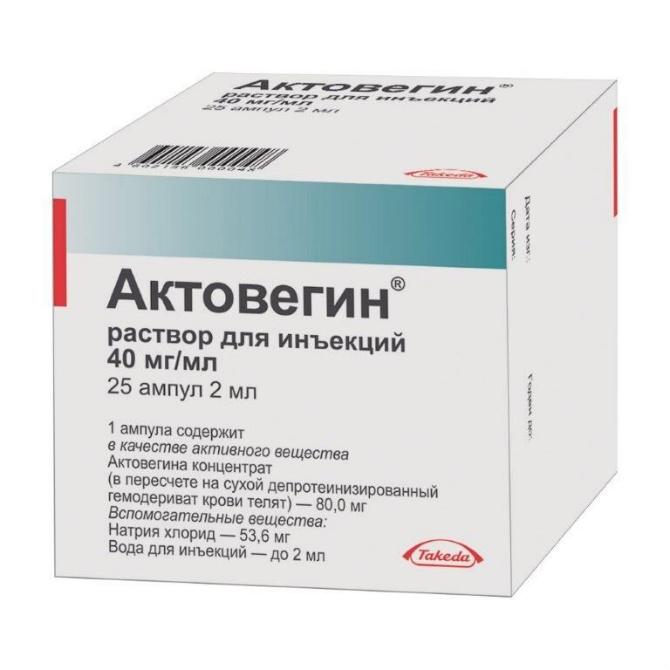
ACTOVEGIN
Release form
Solution for injection
Packing
25 ampoules of 2 ml each
Pharmacological action
Actovegin has a pronounced antihypoxic effect, stimulates the activity of oxidative phosphorylation enzymes, increases the metabolism of energy-rich phosphates, accelerates the breakdown of lactate and beta-hydroxybutyrate normalizes pH, promotes increased blood circulation, intensification of energy-intensive processes of regeneration and repair, improves trophic tissue.
Indications
metabolic and vascular disorders of the brain (including ischemic stroke, traumatic brain injury)
peripheral (arterial and venous) vascular disorders and their consequences (arterial angiopathy, trophic ulcers)
wound healing (ulcers of various etiologies, trophic disorders / bedsores /, burns, disruption of wound healing processes)
prevention and treatment of radiation injuries of the skin and mucous membranes during radiation therapy.
Contraindications
decompensated heart failure
pulmonary edema
oliguria
anuria
fluid retention
hypersensitivity to the components of the
drug hypersensitivity to similar drugs.
Use during pregnancy and lactation
The use of Actovegin during pregnancy did not adversely affect the mother or fetus, however, if it is necessary to use the drug during pregnancy, the potential risk to the fetus should be considered.
Dosage and administration
Injection solution is administered in / a, in / in (including and in the form of infusion) and / m.
Depending on the severity of the clinical picture, the initial dose is 10-20 ml / i / v or i / a, then 5 ml iv or 5 ml i / m is administered daily or several times a week.
When administered in the form of an infusion, 10-20 ml of Actovegin is added to 200-300 ml of the infusion solution (isotonic sodium chloride solution or 5% dextrose solution). The rate of administration is about 2 ml / min.
For metabolic and vascular disorders of the brain, treatment is started with a daily iv injection of 10 ml of an injection for 2 weeks, then 5-10 ml iv 3 4 times a week for at least 2 weeks.
For ischemic stroke, 20-50 ml is diluted in 200-300 ml of the infusion solution and is administered iv drip daily for 1 week, followed by 10-20 ml iv drip for 2 weeks.
With peripheral (arterial and venous) vascular disorders and their consequences, 20-30 ml of the drug is administered in 200 ml of the intravenous or intravenous infusion solution for about 4 weeks.
For wound healing, 10 ml iv or 5 ml iv are administered daily or 3-4 times a week, depending on the healing process (in addition to local Actovegin therapy).
In order to prevent and treat radiation injuries of the skin and mucous membranes, an average of 5 ml iv is administered daily in the intervals between radiation exposure.
In radiation cystitis, 10 ml are administered transurethrally daily in combination with antibiotic therapy.
Side effects
Allergic reactions: skin rash
skin hyperemia
hyperthermia, up to anaphylactic shock.
Drug Interaction
Currently unknown.
However, to avoid possible pharmaceutical incompatibilities, it is not recommended to add other drugs to the Aktovegin ® infusion solution.
Overdose
No information on the overdose of Actovegin has been provided.
Storage conditions
The drug should be stored in a dark place, out of the reach of children, at a temperature not exceeding 25 ° C.
Shelf suitability
3 Year
Deystvuyuschee substances
Deproteynyzyrovann y hemoderyvat blood of lambs
Terms of Pharmacy Leave
Prescription
dosage form
dosage form
injection