Description
Latin name
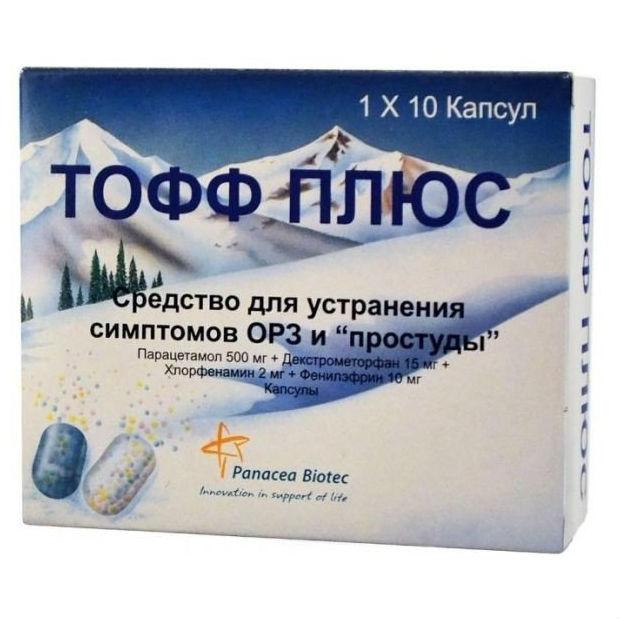
Toff plus
Release form
Toff plus. Capsules
Packing
10 pcs. – blister packagings (1) – packs of cardboard.
Indications
Symptomatic treatment of colds, flu, SARS: Fever.
Pain syndrome.
Rhinorrhea
Contraindications
Hypersensitivity to the individual components of the drug.
Concomitant use of tricyclic antidepressants, monoamine oxidase inhibitors (MAOs), beta-blockers.
Deficiency of glucose-6-phosphate dehydrogenase.
Diseases of the blood.
Hepatic and / or renal failure.
Angle-closure glaucoma.
Prostate hyperplasia.
Gilbert’s syndrome.
Arterial hypertension.
Thyrotoxicosis.
Pheochromocytoma.
Diabetes mellitus.
Bronchial asthma.
Bronchitis.
Pregnancy.
Lactation.
Children under 14 years old.
Precautions: Angina pectoris.
Hypertrophic obstructive cardiomyopathy (GOKMP).
Old age.
Use during pregnancy and lactation
Contraindicated in pregnancy and lactation.
Composition
1 capsule contains:
Active ingredients:
paracetamol 500 mg, chlorpheniramine maleate 2 mg, dextromethorphan hydrobromide 15 mg, phenylephrine hydrochloride 10 mg.
Excipients
Croscarmellose sodium 10.00 mg, povidone (K-30) 20.00 mg, gelatin 5.00 mg, talc 10.00 mg, sodium disulfite 0.50 mg, microcrystalline cellulose 18.64 mg, sucrose 52 , 00 mg, cellulose acetate phthalate 4.50 mg, diethyl phthalate 0.60 mg, quinoline yellow dye 0.13 mg, brilliant blue dye 0.13 mg, crimson dye (Ponceau 4R) 1.50 mg.
Shell composition
Water, gelatin, methyl parahydroxybenzoate, propyl parahydroxybenzoate, sodium lauryl sulfate, brilliant blue dye.
Dosage and Administration
Inside. Adults and children over 14 years of age are prescribed: 1 capsule every 4-6 hours, but no more than 4 capsules per day.
Admission as an antipyretic agent for no more than 3 days without consulting a doctor
Side effects of
From the nervous system and sensory organs: dizziness, drowsiness, increased irritability, mydriasis, paresis of accommodation, increased intraocular pressure.
From the digestive system: dry mouth, epigastric pain, nausea, vomiting, hepatotoxic effect.
From the hemopoietic organs: anemia, thrombocytopenia, hemolytic anemia, aplastic anemia.
From the urinary system: urinary retention, nephrotoxicity (papillary necrosis).
Allergic reactions: skin rash, itching, angioedema, bronchial obstruction.
Other: increase in blood pressure.
Drug Interaction
Enhances the effects of MAO inhibitors, sedatives, ethanol.
Antidepressants, antiparkinsonian agents, antipsychotics, phenothiazine derivatives increase the risk of urinary retention, dry mouth, constipation.
Glucocorticosteroids increase the risk of developing glaucoma.
Paracetamol reduces the effectiveness of uricosuric drugs.
Chlorpheniramine administered concurrently with MAO inhibitors and furazolidone, can lead to the development of hypertensive crisis, psychomotor agitation, fever.
Tricyclic antidepressants increase sympathomimetic activity, and the simultaneous administration of halothane increases the risk of ventricular arrhythmia.
Reduces the hypotensive effect of guanethidine, which in turn enhances the alpha-adrenostimulating activity of phenylephrine.
Overdose
Overdose is usually due to paracetamol, manifested after taking more than 10-15 g of the latter.
Symptoms: pale skin, anorexia, nausea, vomiting, epigastric pain, increased activity of hepatic transaminases, increased prothrombin time, hepatotonecrosis.
Treatment: gastric lavage, activated charcoal, symptomatic therapy.
Storage conditions
In the dark place at a temperature of no higher than 25 ° C.
Expiration
3 years.
Active substance
CHLORPHENAMINE
Dosage
Dosage form
cap uly
Panacea Biotec, India