Description
Release form
Sterile non-hydrogen solution
Pharmacological action
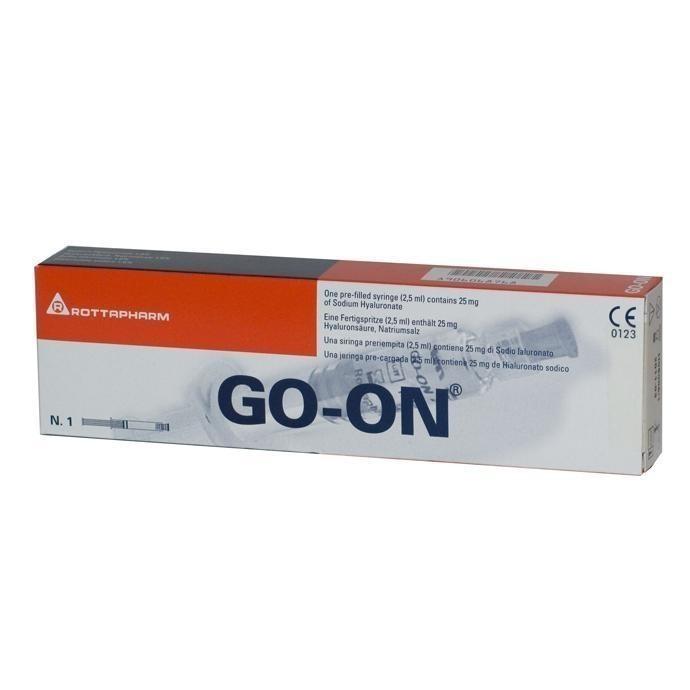
GO-ONR – a sterile non-pyrogenic solution of sodium hyaluronate in a ready-to-use syringe containing 2.5 ml of 1% solution sodium hyaluronate, which is obtained from a Streptococus equi culture by fermentation and subsequent purification.
Indications
for improving the visco-elastic properties of synovial fluid of the knee, shoulder and other joints, for the relief of symptoms of osteoarthritis.
Contraindications
hypersensitivity to one of the components of the drug, inflammatory joint diseases (e.g. rheumatoid arthritis or ankylosing spondylitis).
Special instructions
the drug should be administered directly into the intraarticular space, care should be taken when prescribing the drug to patients with drug allergies, liver failure, elderly patients, children, women during pregnancy during treatment, it is not recommended to breast-feed.
Dosage and administration of
GO-ONR should be administered 5 times at the affected joint at weekly intervals. Several joints can be treated at the same time.
Side effects of
Some patients may experience skin rashes, hives, and itching. In this case, the drug should be discontinued and appropriate treatment prescribed. Administration of GO-ONR may be accompanied by local side effects such as pain, sensation of heat, redness and / or swelling, joint effusion, infection. Such an adverse effect can be removed by applying an ice bladder to the affected joint for 5-10 minutes.
active substance
Hyaluronate Sodium
Terms of delivery from pharmacies
Prescription
dosage form
injection
Rottafarm, Italy