Description
Pharmacological action
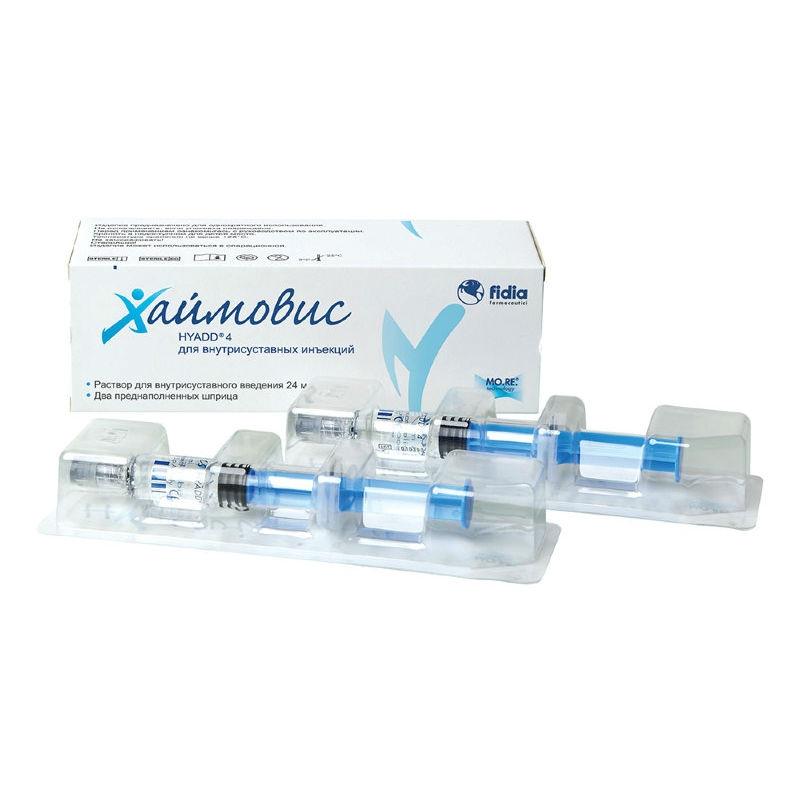
The Haimovis ® preparation is a hydrogel that is manufactured in accordance with the Good Manufacturing Practice (GMP) and contains HYADD ®4, a new molecule, patented by Fidia, at a concentration of 8 mg / ml. HYADD ®4 is a new derivative of hyaluronic acid: partial hyaluronic acid hexadecylamide (partial due to the very low level of modification of hyaluronic acid, at about 3% molar concentration to maintain biocompatibility). This structure gives the Haimovis ® special properties, including long delay times, as well as visco-elastic properties in addition to the excellent lubricating properties of the drug.
Restoring elasticity.
Unlike cross-linked hyaluronic acid, the Haimovis ® preparation has a complete restoration of its elasticity after repeated mechanical stresses, which translates into a more pronounced ability to absorb shock caused by mechanical stress for better joint protection.
Excellent biotribological properties.
Biotribological properties are associated with the ability of a substance to lubricate the surface of cartilage during mutual contact and, thus, protect them from friction and mechanical stress that occurs during movement. Hydrogels may have the same viscosity-elasticity coefficients, but this does not mean that they have the same lubricating effects inside the joint. Himovis shows the best lubricating properties among natural polymers and cross-linked polymers, both in conditions of imitation of healthy cartilage and cartilage with degradation. The physicochemical and biological properties described for the Haimovis preparation are the basis of the clinical efficacy that was found in this drug in the treatment of osteoarthritis and makes it the drug of choice in the treatment of acute and traumatic chondropathy in professional and amateur athletes. Restoring the viscosity of synovial fluid with Haimovis ® effectively reduces pain, restores and preserves joint function in patients with shoulder osteoarthritis. The biomechanical and biological features of Haimovis ® create the prerequisites for expanding the indications for its use in diseases caused by sports.
Indications
For intra-articular administration for injuries and sports medicine
Contraindications
Do not administer to patients with established individual hypersensitivity to the product components or in case of infection or skin diseases at the injection site.
Composition
The main component of the Haymovis medical device 24 mg / 3 ml pre-filled syringe is sodium hyaluronate hexadecylamide (HYADD ®4), obtained by amidation of the sodium hyaluronate carboxyl group with hexadecylamine.
List of starting materials:
– HYADD ®4 (sodium hyaluronate hexadecylamide) – 24 mg
– sodium chloride -25, 5 mg
– sodium hydrogen phosphate dodecahydrate (Na2HP04 x 12H20) – 1.35 mg
– sodium dihydrogen phosphate dihydrate (Na2HP04 x 2H20) – 0.33 mg
– water for injection up to 3 ml (qs).
Dosage and administration
The Haimovis medical device is injected using a sterile needle (18 or 20 gauge) into the affected joint (see Figure 1) once a week, the course of treatment does not exceed 3 weeks. If necessary, repeated courses can be given.
A single procedure usually consists of introducing the Haimovis medical device into 1 large, or 2 medium, or 3-5 small joints. Multiplicity is determined individually: the results of each previous procedure are evaluated, indications for its repetition are established.
Side effects
Local pain, swelling, fever and redness in rare cases may appear at the injection site, such symptoms are usually mild and passing.
More severe inflammatory reactions have been reported with precipitation of sodium pyrophosphate crystals upon intra-articular administration of sodium hyaluronate solutions, although no relationship has been identified.
Failure to observe general precautions and insufficient aseptic processing of the injection site, in rare cases, septic arthritis may occur, as with any intra-articular treatment.
Storage conditions
– Store the medical product in its original packaging at a temperature of 2 ° C.
– Do not freeze.
Shelf life
36 months.
Active ingredient
Sodium hyaluronate
drugstore terms and conditions
prescription
lekarstvennaja form
Solution for