Description
Release form
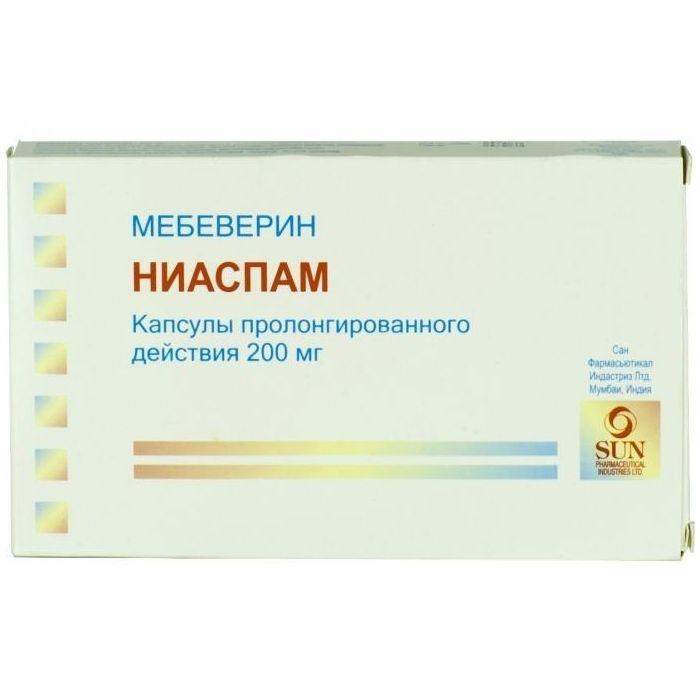
Capsules.
Packaging
in a package of 30 pcs.
Indications
Symptomatic treatment of pain, cramping, dysfunction and discomfort in the intestine associated with irritable bowel syndrome.
Symptomatic treatment of spasms of the gastrointestinal tract (including those caused by organic diseases).
Contraindications
Hypersensitivity to any component of the drug.
Age under 18 (due to insufficient data on efficacy and safety).
Pregnancy (due to insufficient data), period of breastfeeding.
Use during pregnancy and lactation
Pregnancy
There is only extremely limited data on the use of mebeverine by pregnant women. The drug is not recommended for use during pregnancy.
Breastfeeding period
Information on the excretion of mebeverine or its metabolites in breast milk is insufficient. You should not take the drug during breastfeeding.
Fertility
There are no clinical data on the effect of the drug on fertility in men or women, however, animal studies have not demonstrated adverse effects of the drug.
Composition
One sustained-release capsule contains:
active substance: mebeverine hydrochloride 200 mg
excipients: microcrystalline cellulose 65.60 mg, hydrogenated vegetable oil, type I 85.00 mg, purified talc 5.50 mg, magnesium stearate , 00 mg, colloidal silicon dioxide 0.90 mg
capsule shell: gelatin 64.28 mg, purified water 9.00 mg, methyl parahydroxybenzoate 0.52 mg, propyl parahydroxybenzoate 0.13 mg, brilliant blue dye 0.03 mg, titanium dioxide 1.04 mg.
Dosage and Administration
For oral administration.
Capsules must be swallowed with plenty of water (at least 100 ml). Capsules should not be chewed, since their shell provides a sustained release of the drug.
Take one capsule 2 times a day 20 minutes before meals, one capsule in the morning and one capsule in the evening.
The duration of the drug is not limited.
If the patient forgot to take one or more doses, the drug should be continued with the next dose. One or more missed doses should not be taken in addition to the usual dose.
Use in special patient groups
Dosage adjustment is not required for elderly patients, patients with hepatic and / or renal insufficiency.
Side effects
The reports of the side effects listed below were spontaneous and there is insufficient data to accurately assess the frequency of cases.
Allergic reactions were observed mainly from the skin, but there were also other manifestations of allergies.
From the skin and subcutaneous tissues: urticaria, angioedema, including the face, exanthema.
On the part of the immune system: hypersensitivity reactions (anaphylactic reactions).
Drug Interaction
Only studies have been conducted to investigate the interaction of mebeverine with ethanol. Animal studies have shown no interaction between mebeverine and ethanol.
overdose
Symptoms: Theoretically, overdosage of the central nervous system may increase in the event of an overdose. In cases of overdose of mebeverine, the symptoms were either absent or insignificant and, as a rule, rapidly reversible. The noted symptoms of overdose were neurological and cardiovascular.
Treatment: symptomatic treatment is recommended. Gastric lavage is only necessary if intoxication is detected within about one hour after taking multiple doses of the drug. No absorption reduction measures are required. The specific antidote is unknown.
Storage conditions
In a dry, dark place at a temperature of no higher than 25 ° C.
Expiration
2 years.
Deystvuyuschee substances
Mebeverin
dosage form
dosage form
capsules depot
San Pharmaceutical Industries Ltd, India