Description
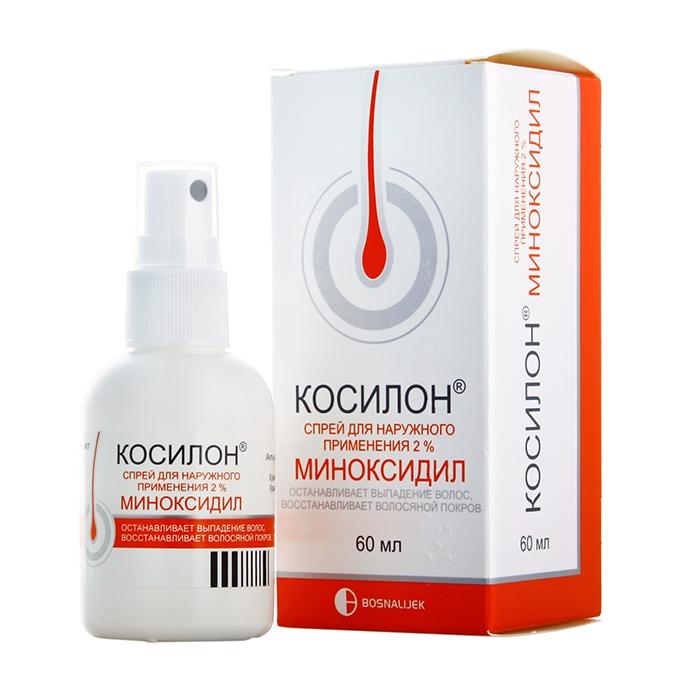
Release form
Solution for external use.
Packaging
Bottle 60 ml.
Pharmacological action
Cosilon has a stimulating effect on hair growth.
Indications
Male pattern baldness (androgenetic alopecia) in men and women.
Contraindications
Hypersensitivity, pregnancy, breast-feeding, violation of the integrity of the skin, dermatosis of the scalp.
Special instructions
Cosilon ® is applied only to dry skin of the scalp after bathing or at least 4 hours before bathing. After applying the drug to the scalp, hands should be thoroughly washed.
It is recommended to wash hair while using Cosilon ® in the usual way. Hairspray and other hair care products can be used during the period of use of Cosilon ®. Before applying hair care products, first apply Cosilon ® and wait until the treated area of the skin is completely dry.
There is no evidence that dyeing hair, perming or using hair softeners may in any way reduce the effectiveness of the drug. However, to prevent possible irritation of the scalp, you must make sure that the drug was completely washed off the hair and skin of the scalp before using these chemicals.
Before starting treatment with Cosilon ®, patients should undergo a general examination, including the collection and examination of a medical history. The doctor must make sure that the scalp is healthy.
If systemic side effects or severe skin reactions occur, patients should discontinue the drug and consult a doctor.
The composition of Cosilon ® includes ethyl alcohol. In case of contact with sensitive surfaces (eyes, irritated skin, mucous membranes), rinse the area with plenty of cold water.
Effect on the ability to drive vehicles and work on machines: The effect of the drug on psychomotor functions has not been noted. The drug does not affect the ability to drive vehicles.
Composition
1 ml contains 20 mg minoxidil.
Dosage and administration
On the dry skin of the scalp, apply 1 ml of Cosilon solution 2 times a day and rub lightly, starting from the center of the affected area.
Side effects
From the skin: hypertrichosis, thinning and increased pigmentation of body hair with topical application – dryness and peeling of the scalp, dermatitis (itching, rash), eczema, increased alopecia, burning sensation of the scalp, folliculitis (sensitivity or soreness at the roots of the hair), erythema
Allergic reactions: rash, swelling of the face, rhinitis, urticaria.
Other: sodium and water retention in the body, swelling, shortness of breath, syncope, decreased libido.
Drug Interactions
There is a theoretical possibility of enhancing orthostatic hypotension in patients receiving concomitant treatment with peripheral vasodilators, but without clinical confirmation.
It is impossible to exclude a very slight increased content of minoxidil in the blood of patients suffering from arterial hypertension and taking minoxidil orally in case of simultaneous use of Cosilon ®, although no relevant clinical studies have been conducted.
It has been established that minoxidil, when applied topically, can interact with some other drugs for external use.
The simultaneous use of a solution of minoxidil for external use and a cream containing betamethasone (0, 05%) leads to a decrease in systemic absorption of minoxidil. The simultaneous application of minoxidil and topical preparations, such as tretinoin and dithranol, to the skin, which cause changes in the protective functions of the skin, can lead to increased absorption of minoxidil.
Overdose of
Accidental ingestion of Cosilon ® can cause systemic side effects due to the vasodilating properties of minoxidil (5 ml of Cosilon ® contains 250 mg of minoxidil, which is 2.5 times the maximum recommended dose for adults when taken in the treatment of hypertension )
Signs of an overdose: fluid retention, decreased blood pressure, tachycardia.
Treatment: to eliminate fluid retention, if necessary, diuretics can be prescribed for the treatment of tachycardia – beta-blockers.
For the treatment of arterial hypotension, a 0.9% sodium chloride solution should be administered intravenously. Symptomatic agents should not be prescribed, for example, norepinephrine and epinephrine, which have excessive pacemaking activity.
Storage Conditions
In a dark place at a temperature not exceeding 25 ° C.
Deystvuyuschee substances
minoxidil
Form of Treatment
spray dlya naruzhnogo primeneniya