Description
Pharmacological action
Phenazopyridine, excreted in the urine, acts on the mucous membrane of the lower urinary tract, where it has a local analgesic effect. This action helps to reduce dysuric phenomena, including pain, burning, frequent urination. The exact mechanism of action is unknown.
Indications
Symptomatic treatment of dysuria (including pain, burning, rapid urination) caused by irritation of the mucous membrane of the lower urinary tract due to infections, injuries, surgical interventions, endoscopic procedures, the use of a probe or catheter.
Directions for use
Inside, after meals, with a full glass of water, do not chew the tablets.
Adults: 2 tablets (200 mg) 3 times a day.
Duration of admission is no more than 2 days (including in combination with antimicrobial agents).
The efficacy and safety of phenazopyridine in children under 18 years of age and in persons over 65 years of age has not been established.
If you accidentally miss a dose of phenazopyridine, you should take it as soon as possible, if the patient remembers an unaccepted dose of the drug in the period immediately before taking the next dose, then you should not double it.
Special instructions
The use of phenazopyridine to relieve symptoms of dysuria due to infection should not delay the diagnosis and initiation of pathogenetic therapy. The drug should be used for symptomatic pain relief, and not to replace specific antimicrobial therapy.
In patients with glucose-6-phosphate dehydrogenase deficiency, phenazopyridine can lead to erythrocyte hemolysis and the development of methemoglobinemia.
When using phenazopyridine, it is possible to stain the urine (with an alkaline reaction) in dark orange or reddish color and feces in orange-red color. A yellowish skin color or sclera may indicate an accumulation of phenazopyridine as a result of impaired renal function or an overdose or taking the drug for more than 2 days, which requires stopping the drug.
Avoid contact lens wear, as phenazopyridine may cause staining of contact lenses.
The duration of phenazopyridine treatment should not exceed 2 days.
2,3,6-triaminopyridine (one of phenazopyridine metabolites) in toxicological studies has demonstrated the ability to damage striated muscle cells and cardiomyocytes. Caution is advised when using phenazopyridine in patients with heart disease and neuromuscular diseases.
Phenazopyridine is contraindicated in patients with any liver disease.
Phenazopyridine is contraindicated in patients with renal failure. It should be borne in mind the possibility of reducing renal function associated with age. Data on the use of phenazopyridine in patients over 65 years of age, including with impaired renal function.
Phenazopyridine can cause changes in the results of urine tests carried out by colorimetric, photometric and fluorimetric methods (determination of ketone bodies, porphyrins, urobilinogen).
If adverse reactions occur, the patient must stop using the drug and consult a doctor immediately.
Impact on driving ability and driving mechanisms
The potential for side effects such as dizziness should be considered. When dizziness occurs, one should refrain from performing the indicated activities.
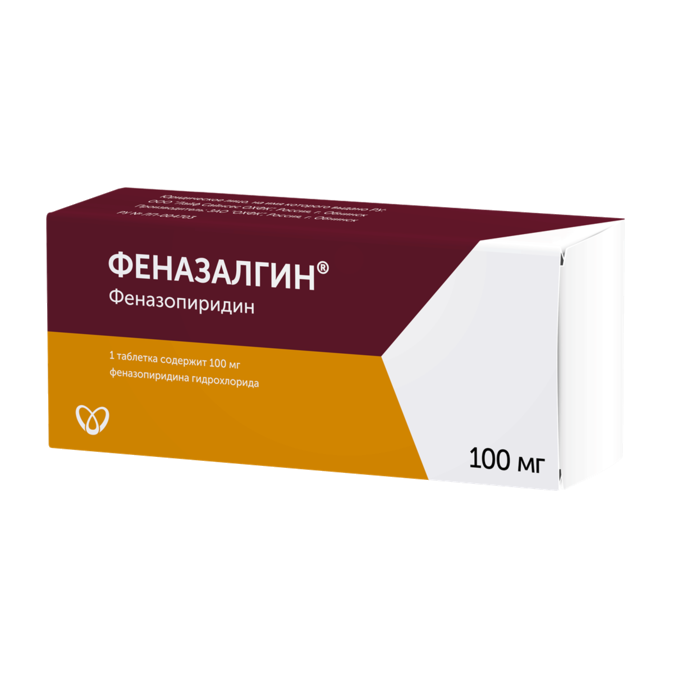
Composition
Brown film-coated tablets, round, biconvex in cross section, brown core with white spots, blushes in the air.
1 tab.
phenazopyridine hydrochloride 100 mg
Excipients: sodium carboxymethyl starch – 26 mg, microcrystalline cellulose – 115.5 mg, colloidal silicon dioxide – 2.5 mg, talc – 3.75 mg, magnesium stearate – 2.25 mg.
Shell composition: hypromellose 6 – 3.9 mg, talc – 1.288 mg, polydextrose – 1.5 mg, titanium dioxide – 1.3 mg, macrogol 3350 – 0.6 mg, dye iron oxide black – 0.279 mg, dye iron oxide yellow – 0.763 mg, dye oxide iron red – 0.37 mg.
Side effects
According to the World Health Organization (WHO) classification, adverse reactions are presented according to their frequency of development: very often ( 1/10) often ( 1/100, <1/10), infrequently ( 1 / 1000, <1/100), rarely ( 1/10 000, <1/1000) and very rarely (<1/10 000), the frequency is unknown - according to available data, it was not possible to establish the frequency of occurrence. From the central nervous system: rarely – headache, dizziness, aseptic meningitis. From the gastrointestinal tract and liver: rarely – nausea, vomiting, diarrhea very rarely – acute hepatotoxicity (associated with an overdose of the drug), jaundice. From the kidneys and urinary tract: uronephrolithiasis, acute nephrotoxicity (associated with an overdose of the drug). On the part of the immune system: rarely – skin rash, itching, fever and other hypersensitivity reactions very rarely – bronchospasm. Allergic reactions: anaphylactoid reaction, allergic hepatitis. From the blood and lymphatic system: methemoglobinemia, hemolytic anemia (with deficiency of glucose-6-phosphate dehydrogenase), sulfhemoglobinemia, neutropenia, leukopenia, pancytopenia. Other disorders: rare – staining of feces in orange-red color, staining of urine in dark orange or reddish color very rarely with prolonged use – change in skin pigmentation and sclera with yellowing, swelling of the face, upper and lower extremities yellowing of nails , visual impairment, eye irritation, earache, reversible loss of color vision. Drug Interactions When taken concomitantly, it may increase the bioavailability of ciprofloxacin. There are no data on other interactions with drugs. If necessary, phenazopyridine may be given in conjunction with antimicrobials. Overdose of Exceeding the recommended dose of phenazopyridine (especially in patients with reduced renal function, as well as in elderly patients) can lead to an increase in its concentration in the blood serum and the development of toxic reactions. Symptoms: methemoglobinemia (especially in patients with glucose-6-phosphate dehydrogenase deficiency), nephro- and hepatoxic manifestations, as well as increased severity of other side effects of the drug. Treatment: discontinue the drug, induce vomiting and take other measures aimed at removing phenazopyridine from the body, as well as symptomatic therapy. To eliminate methemoglobinemia and related symptoms, it is advisable to intravenously administer 1% methylene blue solution (1-2 mg / kg). Storage conditions The drug should be stored out of the reach of children, protected from light at a temperature not exceeding 30 ° C. Shelf life 5 years. Do not use after the expiration date indicated on the package. Terms and conditions by recipe lekarstvennaja form tablets