Description
Latin name
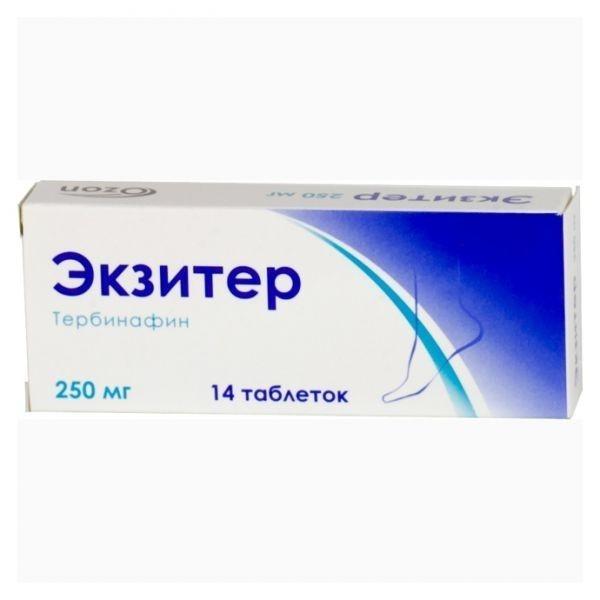
Exiter
release form
tablets
packaging 14 pcs
Indications
Fungal lesions of the skin, nails and hair, candidiasis of the skin and mucous membranes.
Contraindications
Hypersensitivity, severe hepatocellular and renal failure, blood diseases, tumors, metabolic diseases, vascular pathology of the extremities, pregnancy, breast-feeding, children (up to 2 years).
Composition
1 tablet contains:
Active ingredient: terbinafine hydrochloride 281 mg, calculated on terbinafine 250 mg
Excipients: microcrystalline cellulose, sodium starch glycolate, starch gelatinized starch, silica gelatinized.
Dosage and administration of
Inside adults – 250 mg / day in 1 or 2 doses. The duration of treatment depends on the indications and severity of the infection: for skin lesions – 2-4 weeks, for nail damage – from 6 weeks to 4 months or more. For children weighing more than 40 kg – 250 mg / day, 20-40 kg – 125 mg / day, up to 20 kg – 62.5 mg / day.
Topically applied 1-2 times a day for 1-2 weeks.
Side effects of the
Digestive system: nausea, anorexia, moderate abdominal pain, diarrhea, disturbance or loss of taste, cholestasis, jaundice, hepatitis.
Allergic reactions: skin rash rarely – arthralgia, myalgia, erythema multiforme, Stevens-Johnson syndrome.
From the side of the central nervous system: headache.
From the hemopoietic system: neutropenia, thrombocytopenia.
Local reactions: hyperemia, itching, burning sensation.
Storage conditions
In the dark place at a temperature of no higher than 25 ° C. Do not freeze.
Term hodnosty
3 years
Active ingredient
Terbinafine
PMA
tablets
Ozon, Russia