Description
Release form
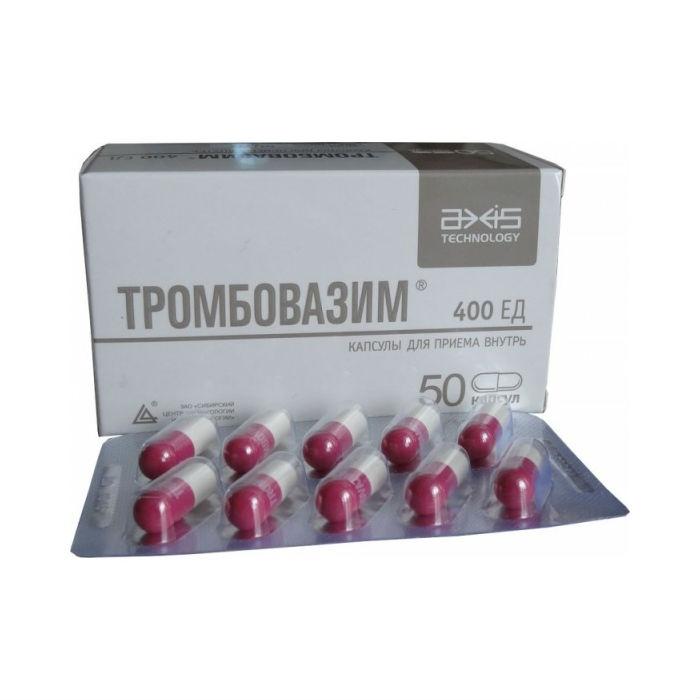
Hard gelatin capsules, No. 0, with a white case and a dark pink cap, with a TROMBOVAZIM white overprint, the contents of the capsules are powder from white with a tan to light brown color.
Pharmacological action
Fibrinolytic agent. It has thrombolytic, anti-inflammatory and cardioprotective effects.
The mechanism of thrombolytic action is associated with direct destruction of fibrin filaments, which form the main thrombus frame. The mechanism of anti-inflammatory action is associated with the influence on the oxidative function of blood neutrophils and tissue macrophages. The mechanism of cardioprotective action is associated with improved blood supply to the myocardium.
Thrombovazim ® is a low-toxic drug.
Thrombovazim ® does not reduce platelet count and does not affect clotting time and bleeding duration.
Indications
As an adjuvant in the treatment of chronic venous insufficiency.
Contraindications
– peptic ulcer of the stomach and duodenum in the exacerbation phase
– pregnancy
– lactation
– under 18 years of age (efficacy and safety not established)
– concomitant use with drugs containing metal salts of divalent zinc, iron) and tetracycline antibiotics (tetracycline, chlortetracycline hydrochloride and oxytetracycline hydrochloride)
– hypersensitivity to the drug.
With caution, the drug should be used in patients with polyvalent allergies, COPD, with the threat of bleeding from the veins of the esophagus, urolithiasis, with peptic ulcer of the stomach and duodenum in remission.
Use during pregnancy and lactation
The drug is contraindicated in pregnancy and lactation (breastfeeding).
Special instructions
Influence on the ability to drive vehicles and control mechanisms
Studies on the possible effect of the drug on the ability to drive vehicles, mechanisms were not carried out, because the drug and its components do not apply to substances that can affect the psychomotor state of a person.
Composition
1 caps. – a complex of proteinases produced by Bacillus subtilis, immobilized by radiation on a macrogol 1500 with the addition of dextran (Thrombovazim) 400 units.
Excipients: potato starch – 261 mg, microcrystalline cellulose – 120 mg, sodium chloride – 9 mg.
Composition of a gelatin capsule: dyes – azorubine (E122), patent blue V (E131), titanium dioxide (E171) gelatin.
Dosage and administration
Take orally 30-40 minutes before meals at a dose of 800-1600 IU per day in 2 doses. The maximum daily dose is 2000 units. The course of treatment is 20 days. In the absence of effectiveness during the course of treatment, the patient should consult a doctor. If necessary, it is possible to conduct repeated courses on the recommendation of a doctor.
Side effects of the digestive system: rarely, dyspeptic symptoms (nausea, vomiting, feeling of heaviness in the stomach).
Others: possibly allergic reactions, a temporary feeling of fullness in the lower extremities.
Drug Interactions
Heparin, dipyridamole, acetylsalicylic acid enhances the antithrombotic effect without increasing the risk of bleeding.
Use with tetracycline antibiotics (tetracycline, chlortetracycline hydrochloride and oxytetracycline hydrochloride) enhances the effect of fibrinolytics.
If it is necessary to use preparations containing salts of divalent metals (calcium, magnesium, zinc, iron) in connection with a possible decrease in the activity of enzymes, it is advisable to observe the interval between doses of Trombovazim ® and these drugs for at least 40 minutes.
Overdose
No overdose was observed with Trombovazim ®.
Storage Conditions
The product should be stored out of reach of children, in a dry, dark place at a temperature not exceeding 25 ° C.
shelf life
2 years
Conditions of sale from drugstores
Without prescription
dosage form
capsules
Prescribing
Prescribing
Adults as prescribed doctor