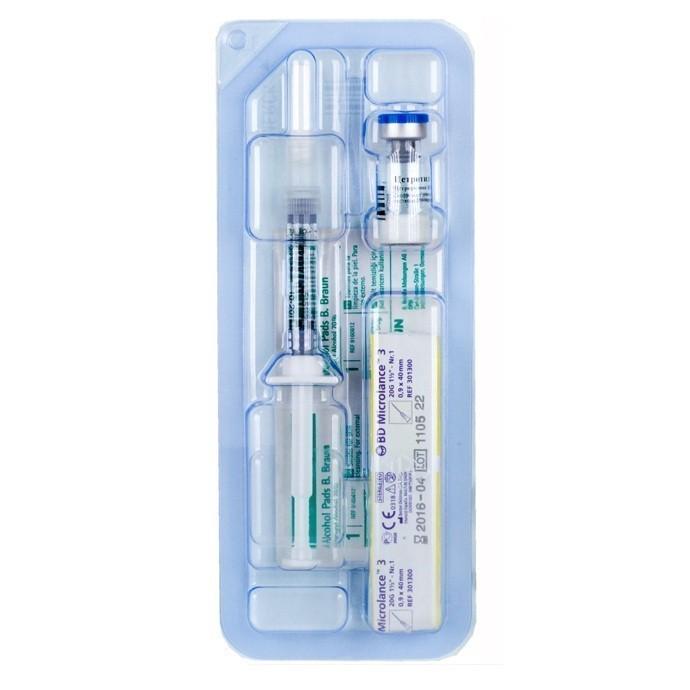
Description
Latin name
CETROTIDE
Release form
Lyophilisate for preparation of solution for subcutaneous administration
Packaging
7 pcs
Pharmacological action
Cetrotide – antigonadotropic.
Pharmacodynamics
Cetrorelix, being an analogue of GnRH, binds to the pituitary cell membrane receptors and competitively inhibits the binding of endogenous GnRH to these receptors. Cetrorelix dose-dependently inhibits the secretion of gonadotropins by the pituitary gland: LH and FSH. In the absence of preliminary stimulation, the onset of inhibition of the pituitary secretory function occurs almost immediately after administration of the drug, the duration of action of cetrorelix depends on the dose administered. In women, cetrorelix causes a delay in raising the level of LH and, therefore, ovulation. After a single injection of 3 mg of cetrorelix, the effect of the drug lasts for at least 4 days (on the 4th day after administration, secretory function is inhibited by 70%). Regular administration of 0.25 mg cetrorelix every 24 hours supports the effect of the drug. The effect of cetrorelix is completely reversible after discontinuation of treatment.
Pharmacokinetics
Absorption and distribution. Quickly absorbed after sc injection, absolute bioavailability is about 85%. Vd is 1.1 l / kg.
Pharmacokinetic parameters after a single sc administration of 0.25 mg and repeated administration (within 14 days), respectively: Cmax in plasma – 4.17–5.92 ng / ml and 5.18–7.96 ng / ml Tmax – 0.5–1.5 h and 0.5–2 h AUC – 23.4–42 ng / h / ml and 36.7–54.2 ng / h / ml.
Withdrawal. T1 / 2 is 2.4–48.8 hours and 4.1–179, 3 hours after a single and multiple (within 14 days) sc administration of a dose of 0.25 mg, respectively. When s / c administration of single doses of cetrorelix (from 0.25 to 3 mg), as well as with daily administration for 14 days, the pharmacokinetics of the drug shows a linear relationship. The average final T1 / 2 after iv and SC administration is 12 and 30 hours, respectively, which indicates absorption at the injection site.
Cetrorelix is excreted by the kidneys. The total plasma and renal clearance are 1.2 ml / min · kg and 0.1 ml / min · kg, respectively. The final T1 / 2 after iv and SC administration is, respectively, an average of about 12 and 30 hours.
Indications
Prevention of premature ovulation in patients with controlled stimulation of ovulation to obtain eggs and assist in reproductive technology.
Contraindications
Hypersensitivity to cetrorelix acetate, mannitol, or other exogenous peptide hormones (drugs similar to Cetrotide).
Pregnancy.
Breastfeeding period.
Postmenopausal period.
Renal failure.
Hepatic failure.
Use during pregnancy and lactation
Cetrotide is not intended for use during pregnancy and during breastfeeding.
Composition
1 vial contains:
Active ingredient: cetrorelix acetate
Excipients: mannitol.
Dosing and Administration
Cetrotide can only be prescribed by a gynecologist. To achieve the maximum effectiveness of the treatment with the drug Cetrotide, you should carefully read these recommendations. After the first injection, the patient should be under medical supervision for 30 minutes to be sure that there is no allergic or pseudo-allergic reaction to the drug. In this case, it is necessary to provide conditions and means for stopping such reactions. Cetrotide at a dose of 0.25 mg (1 vial) should be administered 1 time / day every 24 hours in the morning or in the evening.
Drug administration in the morning: treatment with Cetrotide should begin on the 5th or 6th day of ovarian stimulation (approximately 96 to 120 hours after the start of stimulation) with a gonadotropin preparation, recombinant or excreted from the urine, and continue throughout the entire period of gonadotropin stimulation, including the day of administration of the ovulatory dose of chorionic gonadotropin (CG) .
Drug administration in the evening: treatment with Cetrotide should be started on the 5th day of ovarian stimulation (approximately 96 to 108 hours after the start of stimulation) with a gonadotropin preparation, recombinant or isolated from urine, and continue throughout the entire period of gonadotropin stimulation, including the evening preceding the day of ovulatory administration doses of HCG.
Rules of drug administration: the first injection should be done by a specialist doctor. After receiving the appropriate doctor’s instructions about the symptoms, which may indicate the occurrence of an allergic reaction, the consequences of such a reaction and the need for its treatment, the patient can independently administer Cetrotide. Cetrotide should be administered sc to the lower part of the anterior abdominal wall, preferably in the area around the navel. In order to avoid local irritation during repeated administration of the drug, the injection site should be changed daily. Cetrotide should be dissolved only with the supplied solvent. During dissolution, the bottle must be gently rocked. To avoid bubble formation, vigorous shaking should not be used to speed up dissolution. Do not use the solution if it is opaque or contains undissolved particles. From the vial, draw all its contents into the syringe. This will allow you to enter a dose of cetrorelix of at least 0, 23 mg when using the drug Cetrotide 0.25 mg and at least 2.82 mg when using the drug Cetrotide 3 mg. The solution should be administered immediately after its preparation.
Recommendations for self-administration of the drug: Wash hands. It is very important that your hands and all necessary devices are clean.
On a clean surface, place what is needed for injection (1 vial, 1 syringe with solvent, 1 needle with yellow marking, 1 needle with gray marking and 2 alcohol swabs).
Open the snap-on lid on the vial. Wipe the aluminum ring and rubber stopper with one swab of alcohol.
Remove the wrapper from the yellow marked needle. Remove the solvent syringe from the packaging. Put the needle on the syringe with solvent and remove the protective cap from it.
Insert the needle into the center of the rubber stopper of the vial. Introduce the solution from the syringe into the vial by slowly pressing the piston.
Without removing the needle from the vial, gently shake the vial until the powder is completely dissolved. Avoid vigorous shaking so that no bubbles form during dissolution.
Put the entire contents of the vial into a syringe. If the solution remains in the vial, turn the vial over and extend the needle so that its hole is immediately below the cork. If you look from the side to the inside of the cork, then you can control the movement of the needle and fluid. It is very important to fill the entire contents of the vial into a syringe.
Remove the needle from the syringe and place the syringe. Remove the wrapper from the gray-labeled needle. Put this needle on the syringe and remove the protective cap from it.
Turn the syringe with the needle up and press on the piston until all air bubbles have come out of the syringe. Do not touch the needle or allow it to come into contact with any surface.
The injection site is in the lower part of the anterior abdominal wall, preferably in the area around the navel. Take a second swab soaked in alcohol and wipe the skin at the site of the intended administration. Hold the syringe in one hand, gently squeeze the skin surrounding the injection site with the other hand and firmly fix it between the fingers.
Take the syringe as you normally hold a pencil and insert the needle completely into the skin at a 45 ° angle.
After the needle is fully inserted, the skin should be released.
Gently pull the syringe plunger back. If blood appears in the syringe, proceed in accordance with paragraph 14. In the absence of blood, slowly inject the solution by pressing the piston. After introducing the entire solution, slowly remove the needle and gently press a swab soaked in alcohol on the skin at the injection site. The needle should be removed from the skin at the same angle at which it was inserted.
If blood appears in the syringe, remove the needle from the skin and gently press the injection site with a swab. For repeated injection, this solution can not be used, so the contents of the syringe should be poured. Then start the procedure again from step 1.
The syringe and needles can be used only once. They should be discarded immediately after use (protective caps must be put on the needles to prevent injury).
Drug Interaction
In vitro studies have shown a low likelihood of drug interaction when combined with the administration of Cetrotide with drugs that metabolize with the participation of cytochrome P450 isoenzymes or undergo reactions.
Since it is not possible to completely exclude the possibility of drug interaction with the combined administration of drugs, it is necessary to inform the attending physician about the drugs, taken shortly before treatment with Cetrotide or currently accepted.
overdose
Symptoms: Increased duration of drug, but this is not accompanied by symptoms of acute toxicity.
Treatment: No special measures are required in case of overdose.
Storage conditions
Store at a temperature not exceeding + 25 ° С.
Expiration
2 years.
Deystvuyuschee substances
Tsetrorelyks
Pharmacy
Prescription
dosage form
dosage form
lyophilisates for solution