Description
Packing
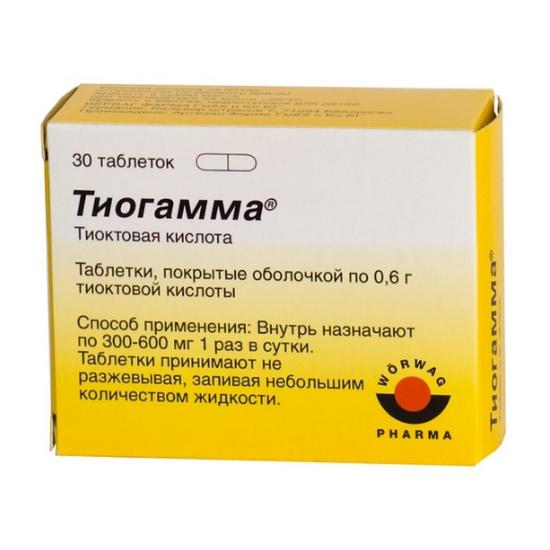
30 pcs
Indications
Diabetic polyneuropathy, alcoholic polyneuropathy.
Contraindications
– Hypersensitivity to the components of the drug
– Pregnancy
– period of breastfeeding.
Clinical data on the use of Thiogamma in children are not available, therefore, children should not be prescribed the drug.
Use during pregnancy and lactation
The drug Thiogamma is contraindicated in pregnancy. If necessary, use the drug during lactation should stop breastfeeding.
In experimental animal studies, no teratogenic effect of thioctic acid was detected. No similar human studies have been conducted.
There is no data on the excretion of thioctic acid with breast milk.
Composition
1 tablet contains:
active substance:
thioctic acid 600 mg
adjuvants:
MCC
lactose
talc
fumed silica
lactose monohydrate
carboxymethylcellulose sodium
simethicone
methylhydroxypropylcellulose (hypromellose)
magnesium stearate
Macrogol 6000
sodium lauryl sulfate
Dosage and administration
Inside, 600 mg are prescribed once a day.
Tablets are taken without chewing with a small amount of liquid.
Side effects
From the nervous system and sensory organs: very rarely – convulsions, diplopia (after iv administration) with rapid administration – increased intracranial pressure.
From the cardiovascular system and blood (hematopoiesis, hemostasis): hemorrhagic rash (purpura), thrombophlebitis (after iv administration).
Allergic reactions: urticaria at the injection site, systemic reactions, up to anaphylactic shock.
Other: hypoglycemia, dyspepsia (when taken orally), after rapid administration, a feeling of heaviness in the head, difficulty in breathing (pass independently) are possible.
Drug Interaction
When used with Thiogamma, it enhances the action of insulin and oral hypoglycemic agents.
When administered with ethanol, the therapeutic efficacy of thioctic acid may be reduced.
Overdose
Symptoms: nausea, vomiting, headache.
Treatment: carry out symptomatic therapy. There is no specific antidote.
Storage conditions
In the dark place at a temperature of no higher than 25 ° C.
Expiration
5 years.
pharmacy leave with prescriptions
dosage form
dosage form
tablets
Dragenofarm Pyushly Apotheker GmbH, Germany