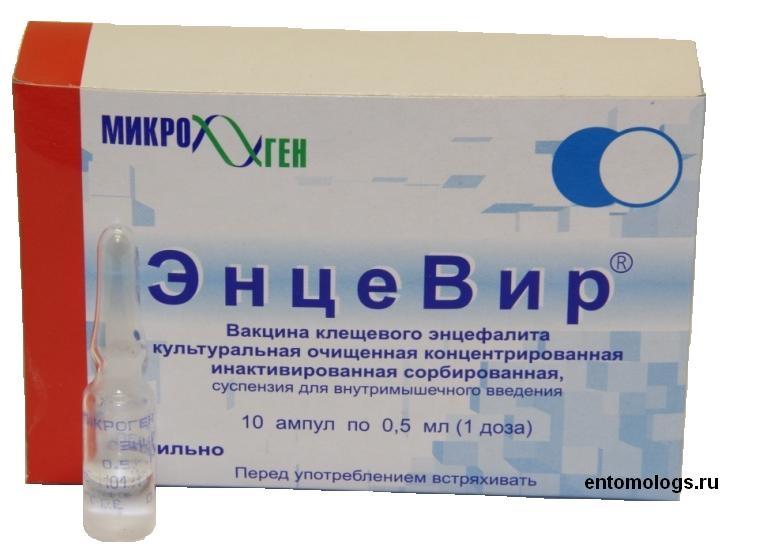
Description
Release form
Suspension for i / m administration
Packing
0.5 ml (1 dose) – ampoules (10) – packs of cardboard.
Pharmacological action of
Vaccine for the prevention of tick-borne encephalitis, obtained by reproduction of tick-borne encephalitis virus in a weighted primary culture of chicken embryonic cells (followed by purification, inactivation with formalin and adsorption on aluminum hydroxide).
Stimulates the production of cellular and humoral immunity to tick-borne encephalitis virus. Provides protection against strains circulating in both Asia and Europe.
Indications
active prophylaxis of tick-borne encephalitis in the following groups of people over 3 years of age: people living in areas enzootic for tick-borne encephalitis, and people who arrived in these territories, performing agricultural, irrigation, land reclamation, construction, procurement, and geological works excavation and displacement, survey, expeditionary, deratization and disinsection persons involved in logging, clearing and improvement of the forest, recreation and recreation areas
active prophylaxis of tick-borne encephalitis in individuals working with live cultures of tick-borne encephalitis
causative agent immunization of donors in order to obtain specific immunoglobulin.
Contraindications
² acute and exacerbation of chronic diseases
² severe allergic reactions with a history of food (especially chicken protein), medicinal substances
² bronchial asthma
² systemic connective tissue diseases
² severe (swelling, hyperemia more than 8 cm in diameter) reaction or complication of previous administration of the
vaccine ² somatic diseases in the sub stage – and
decompensation – epilepsy with frequent seizures
– diabetes mellitus
– thyrotoxicosis and other severe diseases of the endocrine system
– malignant neoplasms
– blood diseases
– pregnancy.
Use during pregnancy and lactation
Vaccination during pregnancy is contraindicated. Vaccination can be done no earlier than than 2 weeks after birth
Special instructions
The vaccine does not contain antibiotics, formaldehyde or preservatives.
The possibility of vaccinating people with various diseases that are not listed in the list of contraindications is determined individually, based on the state of health of the vaccinee and the risk of contracting tick-borne encephalitis. In order to identify contraindications on the day of vaccination, a medical examination and a survey of the vaccineer with mandatory thermometry are carried out.
All cases of unusual reactions after vaccination should be reported to the local health department, State Institute of Standardization and Control of Medical Immunobiological Preparations named after L.A. Tarasevich of the Ministry of Health of Russia (GISK) (121002, Moscow, Sivtsev Vrazhek, 41) with the subsequent provision of medical documentation to the GISK them. L.A. Tarasevich. To the address of GISK them. L.A. Tarasevich also send complaints about the non-compliance of the drug with the specified requirements for physical properties, packaging, packaging.
Composition of
tick-borne encephalitis virus antigen, titer in an enzyme-linked immunosorbent assay at least 1: 1281 dose of
Excipients: aluminum hydroxide (300-500 mcg donor), donor sugar, 300-500 mcg, don (20-30 mg), protamine sulfate (not more than 10 ?g), buffer system (sodium chloride, sodium phosphate disubstituted twelve-water, sodium phosphate monosubstituted two-water), water d / and.
Dosage and administration
The initial course of vaccination is carried out according to the following schemes.
1 scheme
1 vaccination – 0.5 ml on the selected day.
2 vaccination – 0.5 ml after 1-2 months.
3 vaccination – 0.5 ml after 12 months.
2 scheme
1 vaccination – 0.5 ml on the selected day.
2 vaccination – 0.5 ml after 5-7 months.
3 vaccination – 0. 5 ml after 12 months.
Subsequent long-term revaccination is carried out once every 3 years.
When vaccination is carried out during the period of tick activity (in the spring and summer months), contact of the vaccine with the foci of infection should be excluded during the entire vaccination period and 2 weeks after it.
Vaccine administration rules
The vaccine is injected intramuscularly into the deltoid muscle of the shoulder. Intravascular administration of the drug should be avoided. In case of erroneous intravascular administration, reactions can develop up to shock. In such cases, anti-shock therapy should be given immediately.
Immediately prior to injection, the vaccine in the vial is mixed by shaking until a homogeneous suspension is obtained. A separate syringe should be used for each vaccine.
The vaccine should look like a homogeneous, opaque suspension of white color without flakes and impurities. The drug is not suitable for use in ampoules with impaired integrity, labeling, with a color change, the presence of unbreakable flakes, with an expired shelf life, with improper storage.
Vaccination is carried out in vaccination or treatment rooms administered by medical facilities. Nursing staff must have permission to work in the treatment room and to vaccinations, work under the supervision of a doctor. The vaccination room should be equipped with anti-shock therapy.
Vaccination carried out is recorded in the established accounting forms with the date of its administration, dose, vaccine manufacturer, serial number, reaction to vaccination.
Side effects
Local reactions: hyperemia, swelling, pain at the injection site, a slight increase in regional lymph nodes is possible. The duration of the reactions does not exceed 3-5 days.
General reactions: can develop in the first 2 days and include a rise in body temperature from 37.1 ° to 38.0 ° C (9-10%), headache, malaise, pain in muscles and joints. The duration of the reactions does not exceed 3 days.
Other: rarely – allergic reactions (requires medical monitoring within 30 minutes after vaccination).
Storage conditions
Do not freeze the vaccine at a temperature of 2 ° to 8 ° C
Expiration
2 years.
Form of Treatment
suspension dlya inaektsiy
Microgen NPO, Russia