Description
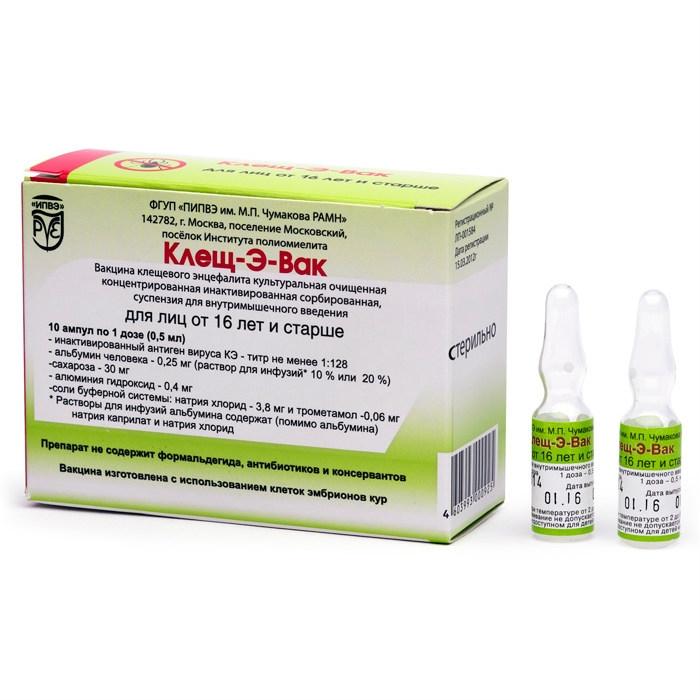
Release form
Suspension for intramuscular administration.
Packing
10 ampoules
Pharmacological action
Pharmacotherapeutic group:
MIBP –
ATX vaccine:
J.07.BA Vaccine for the prevention of encephalitis
J.07.BA 01 Tick-borne encephalitis virus – inactivated whole
Pharmacodynamics:
The vaccine is a purified concentrated suspension of formalin-inactivated tick-borne encephalitis virus (TBE) Sofin strain obtained by reproduction in primary cell culture of chicken embryos sorbed on aluminum hydroxide.
Chicken embryos are received only from healthy poultry from poultry farms, which are safe for infectious diseases of chickens, the quality of the delivered embryos is confirmed by veterinary certificates and certificates of the sanitary status of the livestock laboratory, including microbiological and biochemical controls.
The vaccine contains human albumin (solution for infusion of 10 or 20%). The manufacturer guarantees that the vaccine does not contain antibodies to HIV, HIV-2, to hepatitis C virus and hepatitis B surface antigen, based on documents submitted by the manufacturer of human albumin (registration certificate, analytical passport, certificate of conformity, declaration of conformity).
The vaccine stimulates the production of cellular and humoral immunity to tick-borne encephalitis virus. After two injections of the drug (vaccination course), neutralizing antibodies are detected in no less than 90% of vaccinees.
Indications
– Specific prophylaxis of tick-borne encephalitis for people 16 years and older at a dose of 0.5 ml and for children from 1 year to 16 years at a dose of 0.25 ml
– immunization of donors in order to obtain specific immunoglobulin.
Contingents subject to specific prophylaxis:
1. Population, living in tick-borne encephalitis enzootic areas.
2. Arrivals in these territories carrying out the following work:
– agricultural, irrigation and drainage, construction, excavation and movement of land, harvesting, fishing, geological, surveying, expeditionary, deratization and pest control.
– for logging, clearing and improvement of forests, zones of rehabilitation and recreation of the population.
3. Persons visiting endemic areas for tick-borne encephalitis for recreation, tourism, work in summer cottages and gardens.
4. Persons working with live cultures of tick-borne encephalitis.
Contraindications
1. Acute infectious and non-infectious diseases, chronic diseases in the acute stage – vaccinations are carried out no earlier than 1 month after recovery (remission)
2. Severe allergic reactions in the history of bronchial asthma autoimmune diseases.
3. An allergy to the components of the drug in history.
4. Severe reaction (fever above 40 ° C at the injection site – edema, hyperemia of more than 8 cm in diameter) or complications of the previous dose of the vaccine.
5. Children under 1 year.
When vaccinating donors, the contraindications listed above and the contraindications related to donor selection should be considered.
In each case of a disease not included in this list of contraindications, vaccination is carried out with the permission of the doctor, based on the health status of the vaccinee and the risk of contracting tick-borne encephalitis. In order to identify contraindications, the doctor (paramedic) conducts a survey and examination of the vaccinee with mandatory thermometry on the day of vaccination.
Use during pregnancy and lactation
Clinical studies of the safety of the Mite-E-Wak vaccine for pregnant and lactating women have not been conducted.
Vaccination of pregnant women can be carried out only after a thorough determination of the risk of their possible infection with the TBE virus.
Vaccination of nursing women can be done 2 weeks after delivery.
Special instructions
Vaccinations are carried out with strict adherence to aseptic and antiseptic rules. The room should be equipped with anti-shock and anti-allergic therapy.
Before opening the ampoule, it is necessary to conduct a visual inspection. The drug is not suitable in ampoules with impaired integrity, labeling, if foreign impurities are found, in the presence of large, non-breaking conglomerates, with an expired Expiration, or in violation of the temperature regime of storage or transportation.
Immediately before injection, the vaccine in the ampoule is shaken until a homogeneous suspension is obtained. The drug is administered immediately after opening the ampoule intramuscularly into the deltoid muscle of the shoulder.
Vaccinations carried out are recorded in the established accounting forms with the name of the drug, the date of vaccination, doses, batch numbers, manufacturer’s plants, vaccine response.
The drug can not be administered intravenously!
Vaccination of children and adults with chronic diseases in the acute stage is carried out no earlier than 1 month after recovery (remission).
The vaccine is not used for children under 1 year old.
Impact on the ability to drive transp. Wed and fur .:
Pronounced general reactions to the vaccine (a significant increase in temperature, severe headache) are a contraindication for driving vehicles and mechanisms.
Composition of
One vaccination dose for people 16 years of age and older (0.5 ml) contains:
active ingredient: inactivated antigen of KE virus – titer of at least 1: 128
excipients: human albumin (solution for infusion * 10% or 20%) 0.25 mg sucrose 30 mg aluminum hydroxide 0.4 mg salts of the buffer system: sodium chloride 3.8 mg, trometamol 0.06 mg.
Side effects of
After administration of the vaccine, local and general reactions may develop in some cases.
When evaluating adverse reactions of the drug, the following frequency data formed the basis:
very often> 10%
often – from 1 to 10%
occasionally – from 0.1 to 1%
rarely – from 0.01 to 0 , 1%
is very rare – <0.01%, including isolated cases. For persons 16 years of age and older Local reactions: often – redness, swelling, soreness at the injection site is very rare – the development of infiltrate, and also in very rare cases – a slight increase in regional lymph nodes. Local reactions may occur within 2 days after vaccination. The duration of local reactions does not exceed 3 days. General reactions: often – general malaise, headache, nausea, fever (up to 37.5 ° C (mild reaction) – often from 37.5 ° C to 38.5 ° C (medium reaction) – from case to case over 38.5 ° C (strong reaction) – rarely). General reactions can develop within 2 days after vaccination, their duration does not exceed 2 days. Drug interaction It is allowed to vaccinate against tick-borne encephalitis at the same time (on the same day) with other vaccinations with inactivated vaccines of the National preventive vaccination calendar and the vaccination calendar for epidemic indications (except for rabies). In other cases, vaccination against tick-borne encephalitis is carried out no earlier than 1 month after vaccination against another infectious disease. Overdose There have been no cases of overdose. Very rarely vaccinations can be accompanied by the development of allergic reactions of an immediate type, in connection with which vaccines should be under medical supervision for 30 minutes after vaccination. Vaccination sites should be provided with anti-shock therapy. Storage Conditions The drug is stored and transported at 2 to 8 ° C. Short-term (max. 24 hours) transportation at 9 ° C to 20 ° C is permitted. Freezing is not allowed. Keep out of the reach and sight of children. Expiration 2 years. A drug that has expired cannot be used. Terms leave through pharmacies In retseptu FSUE Institute Chumakov, Russia