Description
Latin name
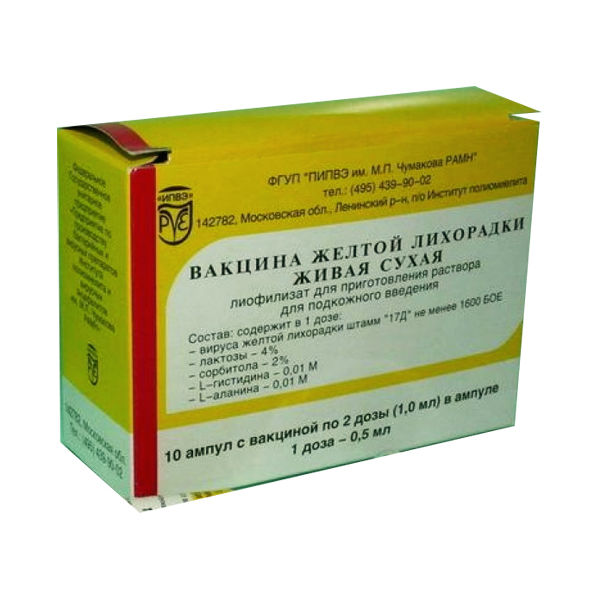
YELLOW FEVER VACCINE, LIVE, DRY
Release form
Lyophilisate for preparation of solution for SC administration
Packaging
10 vials
Indications
Prevention of yellow fever in children from 9 months of age and adults traveling abroad in yellow fever enzootic areas, as well as in individuals working with live cultures of yellow fever.
Contraindications
1. Acute infectious and non-infectious diseases, chronic diseases in the acute stage or decompensation – vaccinations are carried out no earlier than one month after recovery (remission).
2. A history of allergic reaction to egg white protein.
3. Primary (congenital) immunodeficiencies.
4. Secondary (acquired) immunodeficiencies: treatment with immunosuppressants, antimetabolites, radiotherapy – vaccinations are carried out no earlier than 12 months after recovery (end of treatment).
5. Pregnancy.
In order to identify contraindications, the doctor conducts a survey and examination of vaccinees with mandatory thermometry on the day of vaccination. A specific decision on the need for vaccinations for certain groups of people (pregnant women, patients with chronic diseases, malignant blood diseases and neoplasms, etc.), living in enzootic areas with yellow fever, depends on the degree of risk of the disease.
Use during pregnancy and lactation
Contraindicated in pregnancy.
Composition
Yellow fever vaccine is a fine tissue of chicken embryos free of specific pathogen microflora (specific pathogen free-SPF) infected with attenuated yellow fever virus strain 17D, purified by centrifugation and lyophilized.
One dose of a solution for subcutaneous administration is 0.5 ml of the reconstituted preparation and contains: yellow fever virus of at least 1000 LD50 or 1600 PFU – active component excipients: lactose (monohydrate) 20.0 mg – stabilizer, sorbitol 10.0 mg – stabilizer, L-histidine 1.2 mg – stabilizer, L-alanine 0.7 mg – stabilizer. The drug does not contain preservatives or antibiotics.
Dosage and administration
Vaccination is carried out once subcutaneously, with a syringe at the external angle of the scapula or in the region of the deltoid muscle of the shoulder at a dose of 0.5 ml for all age groups no later than 10 days before departure to the enzootic area. Revaccination if necessary, carried out 10 years after vaccination with the same dose.
The drug is not suitable for use in ampoules with impaired integrity and labeling, when physical properties change (deformation of the tablet – the light pink porous mass changes shape and sharply decreases in volume, the inhomogeneity of the dissolved drug, etc.), with an expired shelf life, when violation of the temperature regime of storage and transportation.
Opening of ampoules and the vaccination procedure is carried out with strict observance of the rules of asepsis and antiseptics.
All contents of the ampoule with solvent are used to dissolve the vaccine. The vaccine should be completely dissolved within 5 minutes. The dissolved vaccine is an opalescent yellowish-pink liquid. The dissolved vaccine can withstand 10-15 minutes, then the vial is shaken and a single inoculative dose of 0.5 ml is injected into the syringe. It is allowed to store the dissolved vaccine closed with a sterile cloth for no more than 1 hour at a temperature of 2 to 8 ° C.
The vaccine administered is recorded in the prescribed registration forms, indicating the name of the drug, date of vaccination, dose, batch number, reaction to vaccination.
Side effects of
After administration of the vaccine, local and general reactions may develop in some cases.
Local reaction manifests itself as hyperemia and edema (with a diameter of not more than 2.5 cm), which can appear after 12-24 hours and disappears 2-3 days after the injection. In extremely rare cases, compaction of the subcutaneous tissue develops, accompanied by itching, pain, an increase in regional lymph nodes.
General reaction may develop between 4 and 10 days after vaccination in the form of fever up to 38.5 ° C, malaise, dizziness, headache, chills. The duration of the general reaction does not exceed 3 days.
In rare cases, allergic complications are possible. In this regard, the vaccination points should be equipped with anti-shock therapy, and the vaccinated must be under medical supervision for 30 minutes after vaccination.
To persons predisposed to allergic reactions, prescribe antihistamines for 2-4 days before and after vaccination.
Drug Interactions
For people over 15 years of age it is allowed to vaccinate against yellow fever at the same time (on the same day) with other vaccinations of the national calendar of vaccinations, provided that the drugs are administered in different parts of the body.
For children under 15 years of age, the interval between the previous vaccination against another infection and vaccination against yellow fever should be at least 2 months.
The use of yellow fever vaccine is contraindicated simultaneously with vaccines intended for the prevention of cholera and paratyphoid A and B. In this case, an interval of 3 months should be observed between both vaccinations.
Storage Conditions
The vaccine is stored at 2 to 8 ° C.
The solvent is stored at 2 to 25 ° C. Freezing of the solvent is not allowed.
Keep out of the reach and sight of children.
Transport conditions
Transport in accordance with SP 3.3.2.2329-08 at 2 to 8 ° C in thermocontainers.
Expiration
The vaccine is 2 years. Vaccines that have expired should not be used.
Solvent – 4 years.
lekarstvennaja form
Solution for
Federal State Unitary Enterprise Institute im Chumakova, Russia