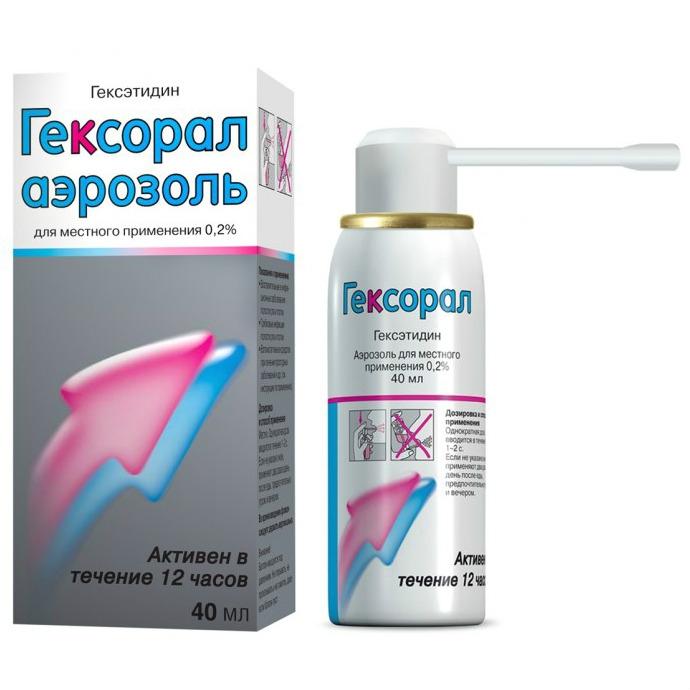
Description
Release form
Topical aerosol 0.2%.
40 ml of the drug in an aluminum aerosol can with an internal varnish coating.
For 1 aerosol can complete with one spray nozzle or four spray nozzles of different colors along with instructions for use in a cardboard box.
Pharmacological action
Pharmacotherapeutic group:
antiseptic. ATX code: A01AB12.
Pharmacodynamics
The antimicrobial effect of the drug Hexoral ® is associated with the suppression of oxidative reactions of bacterial metabolism (thiamine antagonist). The drug has a wide spectrum of antibacterial and antifungal effects, in particular against gram-positive bacteria and fungi of the genus Candida, however, the preparation Hexoral ® can also have an effect in the treatment of infections caused, for example, by Pseudomonas aeruginosa or Proteus spp.
At a concentration of 100 mg / ml, the drug suppresses most bacterial strains. The development of sustainability was not observed. Hexetidine has a weak anesthetic effect on the mucous membrane. The drug has an antiviral effect against influenza A viruses, respiratory syncytial virus (PC virus), herpes simplex virus type 1, affecting the respiratory tract.
Pharmacokinetics
Hexatidine adheres very well to the mucous membrane and is practically not absorbed. After a single use of the active substance, its traces are found on the gum mucosa for 65 hours. In plaque, active concentrations remain for 10-14 hours after application.
Contraindications
– Hypersensitivity to any of the components of the drug
– erosive-desquamous lesions of the oral mucosa
– children under 3 years of age.
Use during pregnancy and lactation
There is no information on any undesirable effects of the drug ² ÑHexoral ® ² Ñ during pregnancy and during breast-feeding.
However, before prescribing Hexoral ® to pregnant or lactating women, the doctor must carefully weigh the benefits and risks of treatment, given the lack of sufficient data on the penetration of the drug through the placenta and into breast milk.
Composition
100 ml of the preparation contain:
active substance
– hexetidine – 0.200 g
excipients:
polysorbate 80 – 1.400 g,
citric acid monohydrate – 0.070 g,
sodium saccharinate – 0.040 g,
levomenthol – 0.070 g,
eucalyptus rod leaves oil – 0.0011 g,
sodium calcium edetate – 9600 g, 0.1l g % – 4.333 g,
sodium hydroxide – qs to pH 5.5 ± 0.2,
purified water – qs to 100 ml,
nitrogen – qs up to 5 bar.
Dosage and Administration
Topically.
Children from 3 to 6 years: the use of the drug is possible after consultation with a medical professional.
Adults and children over 6 years of age: treat affected areas with respiratory arrest, 1 injection for 1-2 seconds 2 times a day.
Hexatidine adheres to the mucous membrane and therefore gives a lasting effect. In this regard, the drug should be used after meals.
Side effects of
Adverse reactions detected during post-registration use of the drug were classified as follows: very frequent (1/10), frequent (1/100, <1/10), not frequent (1/1000, <1 / 100), rare (1/10000, <1/1000), very rare (<1/10000), the frequency is unknown (the frequency of occurrence cannot be estimated based on the available data). Immune system disorders. Very rarely: hypersensitivity reactions (including urticaria), angioedema. Disorders of the nervous system. Very rarely: ageusia, dysgeusia. Disorders of the respiratory system, chest and mediastinal organs. Very rarely: cough, shortness of breath due to the appearance of a hypersensitivity reaction. Disorders of the gastrointestinal tract. Very rarely: dry mouth, dysphagia, nausea, increased salivary glands, vomiting. General disorders and disorders at the injection site. Very rarely: reactions at the site of application (including irritation of the mucous membrane of the oral cavity and pharynx, burning sensation, paresthesia of the oral cavity, discoloration of the tongue, discoloration of the teeth, inflammation, blistering and ulceration). If any of the side effects indicated in the instructions are aggravated or you notice other side effects, it is recommended to consult a doctor. Overdose of It is unlikely that hexetidine can have a toxic effect when used according to the instructions for use of the drug. Ingestion of a large amount of an ethanol-containing preparation may result in signs / symptoms of alcohol intoxication. In case of any overdose, consult a doctor immediately. Treatment is symptomatic, as with alcohol intoxication. Gastric lavage is necessary within 2 hours after swallowing an excess dose. Storage conditions Store at a temperature not exceeding 25 ° C. Keep out of the reach of children. Expiration 3 years. Do not use the drug after the expiration date indicated on the package. The contents of the aerosol can should be used within 6 months after the first use. Deystvuyuschee substances HeksÑtydyn Terms and conditions Without a prescription Dosage form spray for oral cavity