Description
Release form
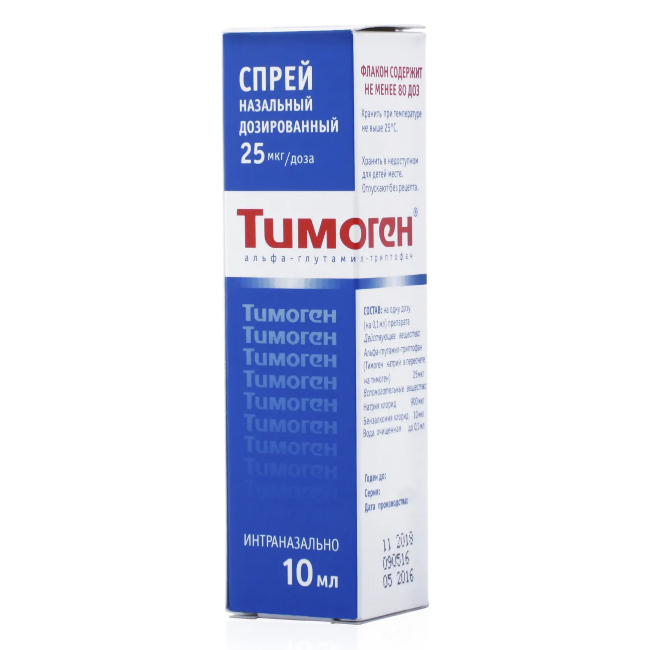
Dosage nasal spray 25 Ñg / dose of 10 ml in a dark glass bottle or polymer bottle. A metering pump (100 Ñl) is installed in the bottle with a plastic case, nozzle and protective cap. One bottle with instructions for use in a pack of cardboard. The number of doses in the bottle is at least 80.
Pharmacological action
Pharmacotherapeutic group: Immunostimulating agent.
ATX Code: L03AX.
Pharmacological properties:
Pharmacodynamics: It has a regulatory effect on the reactions of cellular, humoral immunity and nonspecific resistance of the body. Stimulates regeneration processes in the event of inhibition. Improves the course of cellular metabolism. It enhances the expression of differentiation receptors on lymphocytes, normalizes the number of T-helpers, cytotoxic T-lymphocytes and their ratio in patients with various immunodeficiency states.
Pharmacokinetics: The drug quickly enters the systemic circulation after its intranasal administration. Peptidase alpha-glutamyl-tryptophan is cleaved by L-glutamic acid and L-tryptophan, which are involved in peptide synthesis in the body.
Indications
Prevention and treatment of acute and chronic viral and bacterial diseases of the upper respiratory tract.
Prevention of suppression of immunity, blood formation, regeneration processes in the post-traumatic and postoperative periods.
Comprehensive adjuvant therapy to correct secondary immunodeficiency in radiation therapy, chemotherapy and antibiotic therapy.
Combined therapy of acute and chronic infectious and inflammatory diseases accompanied by a decrease in immunity.
If necessary, please consult your doctor before taking the drug.
Contraindications
Hypersensitivity to the drug, children under 1 year old. If necessary, please consult your doctor before taking the drug.
Use during pregnancy and lactation
Due to insufficient clinical data, taking the drug during pregnancy and during breastfeeding is possible only as directed by a doctor if the intended benefit to the mother outweighs the potential risk to the fetus or baby. Please consult your doctor before taking the drug.
Special instructions
The drug does not have a specific effect on the first use and when it is canceled. There are no specific features of the use of the drug in children and adults with chronic diseases.
Information about the possible effect of a medicinal product for medical use on the ability to drive vehicles, mechanisms:
The drug does not adversely affect the ability to drive vehicles and engage in other potentially dangerous activities that require an increased concentration of attention and speed of psychomotor reactions.
Composition of
In one dose (0.1 ml) of the drug
Active ingredient: alpha-glutamyl-tryptophan (Timogen sodium in terms of thymogen) 25 mcg.
Excipients: sodium chloride 900 mcg, benzalkonium chloride 10 mcg, purified water to 0.1 ml.
Dosage and Administration
Topically.
Hold the bottle with the thumb on the base, and the nozzle of the metering device between the middle and index fingers. Insert the nozzle of the dosing device into the nasal passage in a vertical position. Press nozzle once.
The drug is administered intranasally (in the nose) in doses: Adults: 1 dose (one injection) in each nasal passage 2 times a day for 10 days for therapeutic purposes or for 3-5 days for prophylactic purposes.
For children: from 1 year to 6 years, 1 dose in one nasal passage 1 time per day, from 7 to 14 years, 1 dose in each nasal passage 1 time per day. The drug is used for 10 days for therapeutic purposes or for 3-5 days for prophylactic purposes. A repeated course of Timogen is carried out not earlier than after 1 month.
Take the drug only according to the method of use and at the doses indicated in the instructions. If necessary, please consult your doctor before taking the drug.
Side effects
Allergic rhinitis. Consult a doctor if you have the mentioned side effect or other side effects not listed in the instructions.
Drug Interactions
Increases the effectiveness of chemotherapeutic drugs (cytostatic, antibacterial drugs) and radiation therapy. It is not recommended to use simultaneously with large doses of glucocorticosteroid drugs. The immunosuppressive effect of large doses of these drugs eliminates the immunostimulating effect of alpha-glutamyl tryptophan.
Overdose of
In case of overdose, there is a copious secretion of nasal mucous membrane secretion, which stops after drug withdrawal. No treatment required.
If you accidentally take the drug inside, you need to rinse your stomach with water and consult a doctor immediately.
Storage Conditions
Store at 2 ° C to 8 ° C. Keep out of reach of children.
Expiration
2 years 6 months. Do not use after the expiry date, decreea nd on the packaging.
Dosage form
spray nasal
Tsitomed MBNPK, Russia