Description
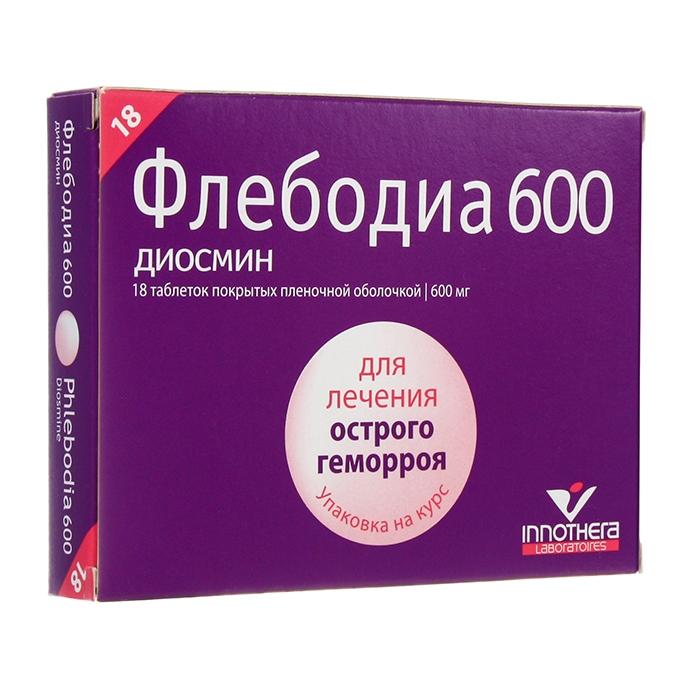
Release form
Film-coated tablets.
packaging 18 pcs per pack
Pharmacological action of
The drug has a phlebotonizing effect (reduces the extensibility of veins, increases venous tone (dose-dependent effect), reduces venous congestion), improves lymphatic drainage (increases the tone and frequency of contraction of lymphatic capillaries, increases their functional density, reduces lymphatic pressure), improves lymphatic pressure), improves microcirculation (increases capillary resistance (dose-dependent effect), reduces their permeability), reduces the adhesion of leukocytes to the venous wall and their migration to steam venous tissue, improves oxygen diffusion and perfusion in the skin tissue, has an anti-inflammatory effect. Enhances the vasoconstrictor effect of adrenaline, norepinephrine, blocks the production of free radicals, the synthesis of prostaglandins and thromboxane.
Indications
As part of complex therapy:
– to eliminate the symptoms of lymphovenous insufficiency of the lower extremities: a feeling of heaviness or fatigue in the legs, pain
– additional treatment for impaired microcirculation: – symptomatic treatment of acute hemorrhoids.
Contraindications
Hypersensitivity to the drug, breast-feeding, children under 18 years of age, pregnancy (I trimester) (limited experience).
Use during pregnancy and lactation
Until now, in clinical practice there have been no reports of any side effects when using the drug in pregnant women. The use during pregnancy in the II and III
trimesters is possible only as prescribed by the doctor in cases where the expected benefit to the mother outweighs the potential risk to the fetus.
In experimental studies, no teratogenic effects on the fetus were detected. During breastfeeding, it is not recommended to take the drug, because there is no data on the penetration of the drug into breast milk.
Special instructions
Treatment of an acute attack of hemorrhoids is carried out in combination with other drugs, in the absence of a quick clinical effect, an additional examination and adjustment of the therapy are necessary.
Influence on the ability to drive vehicles
There is no evidence of a negative effect of the drug on the ability to drive vehicles and other mechanisms.
Composition
Active ingredient:
Diosmin in terms of dry matter …………. 600 mg
Excipients:
talc …….. ……………………………………. 10.24 mg
silicon dioxide colloidal ………………… 3.5 mg
stearic acid ………………… ……….. 50.05 mg
microcrystalline cellulose ………… up to 910 mg
Film composition:
Sepiphilm® 002 (hypromellose (E 464) – 9.832 mg, microcrystalline cellulose -7.866 mg, macrogol 8 stearate type 1 – 1.967 mg). Sepisprs® AR 5523 pink (propylene glycol – traces, hypromellose (E 464) – 0.458 mg, titanium dioxide (E 171) -4.026 mg, crimson dye [Ponceau 4R] (E 124) – 0.401 mg, black iron oxide (E 172) 0.130 mg iron oxide red (E 172) – 0.020 mg).
Opaglos 6000 (carnauba wax (E 903) – 0.075 mg, beeswax (E 901) – 0.075 mg, shellac (E 904) – 0.150 mg, ethanol 95 ° – traces).
Dosage and administration of
Before using the drug, consult your doctor.
The drug is intended for oral administration.
With varicose veins of the lower extremities and chronic lymphovenous insufficiency (edema, pain, cramps), I tablet is prescribed per day in the morning on an empty stomach. The duration of therapy is usually 2 months. With exacerbation of hemorrhoids, the drug is prescribed for 2-3 tablets per day with meals for 7 days. If one or more doses of the drug is missed, it is necessary to continue the use of the drug in the usual mode and in the usual dose.
Side effects of
In rare cases, hypersensitivity to the components of the drug, requiring a break in treatment: from the gastrointestinal tract – dyspeitic disorders (heartburn, nausea, abdominal pain), from the central nervous system – headache.
If any of the side effects indicated in the instructions are aggravated, or you notice any other side effects not listed in the instructions, inform your doctor.
Drug Interactions
No clinically significant effects of interaction with other drugs have been described.
Overdose
Overdose symptoms are not described.
Storage conditions
Do not store above 30 ° C.
Keep out of the reach and sight of children.
Shelf life
3 years. Do not use after expiration date.
dosage form
tablets
Prescribing
Prescribing
Prescribing
for the physician, Adults as prescribed by the doctor