Description
Latin name
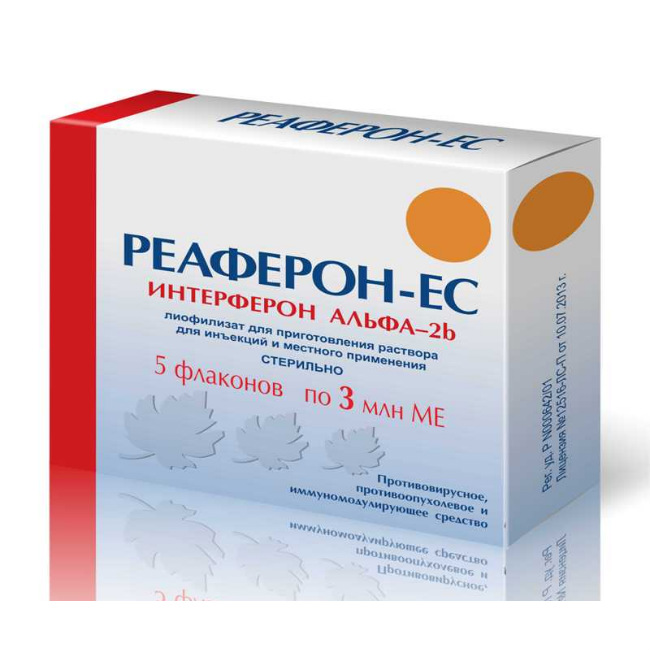
REAFERON-EC
release lyophilisate for solution for injection and places th use in the form of a powder or porous mass of white, hygroscopic formed colorless transparent or slightly opalescent solution upon dilution.
Pharmacological action
The drug has antiviral, antitumor, immunomodulating activity.
Interferon alpha-2b human recombinant, which is the active ingredient in the preparation, is synthesized by bacterial cells of the strain Escherichia coli SG-20050 / pIF16. in the genetic apparatus of which the human interferon alpha-2b gene is integrated. It is a protein containing 165 amino acids, and is identical in characteristics and properties to human leukocyte interferon alpha-2b.
The antiviral effect of interferon alpha-2b is manifested during the reproduction of the virus by active inclusion in the metabolic processes of cells. Interferon, interacting with specific receptors on the cell surface, initiates a number of intracellular changes, including the synthesis of specific cytokines and enzymes (2-5-adepilatsynthetaza and protein kinases), the action of which inhibits the formation of viral protein and viral ribonucleic acid in the cell. The immunomodulatory effect of interferon alpha-2b is manifested in an increase in the phagocytic activity of macrophages, an increase in the specific cytotoxic effect of lymphocytes on target cells, and a change in the quantitative and qualitative composition of secreted cytokines. a change in the functional activity of immunocompetent cells, a change in the production and secretion of intracellular proteins. The antitumor effect of the drug is realized by suppressing the proliferation of tumor cells and the synthesis of certain oncogenes, leading to inhibition of tumor growth.
Indications
Adults in complex therapy:
– acute viral hepatitis B – moderate and severe forms at the beginning of the icteric period until the 5th day of jaundice (in later terms, the drug is less effective if the drug develops hepatic coma and
cholestatic course of the disease)
– acute prolonged hepatitis B and C, chronic active hepatitis B and C, chronic hepatitis B with a delta agent, without signs of cirrhosis and with signs of liver cirrhosis
– stage IV kidney cancer, hairy cell leukemia, malignant lymphomas of the skin (fungal mycosis, primary reticulosis, reticulosarcomatosis), Kaposi s sarcoma, basal cell carcinoma
skin, keratoacanthomas, chronic myeloid leukemia, histiocytosis from Langerhans cells, subleukemic myelosis,
essential thrombocythemia
– viral conjunctivitis, keratoconjunctivitis, keratitis, keratoiridocycle one keratouveitis.
In complex therapy in children from 1 year of age:
– with acute lymphoblastic leukemia in the remission period after induction chemotherapy (at 4-5 months of remission)
– with respiratory laryngeal papillomatosis, starting from the day after the removal of papillomas.
Contraindications
– hypersensitivity to the components of the drug
– severe allergic diseases
– severe diseases of the cardiovascular system: heart failure in the decompensation stage, recent myocardial infarction, severe cardiac arrhythmias
– severe or hepatic including caused by the presence of metastases, chronic hepatitis with uncompensated liver cirrhosis, autoimmune hepatitis
– epilepsy and other disorders of the central nervous system, mental illness and disorder in children and adolescents
– an autoimmune disease with a history of
– the use of immunosuppressants after transplantation
– thyroid diseases that cannot be controlled by conventional therapeutic methods
– CC below 50 ml / min (when prescribed in combination with ribirdine) when used in combination with ribavirin, the contraindications indicated in the instructions for use of ribavirin
should also be taken into account – use in men whose partners are pregnant
– pregnancy s and during breastfeeding.
With caution
is renal and / or liver failure, severe myelosuppression.
– mental disorders, especially expressed by depression, suicidal thoughts and attempts in history.
– for patients with psoriasis, sarcoidosis.
Use during pregnancy and lactation
The drug is contraindicated for use in the III trimester of pregnancy. In the first and second trimesters, the drug should be used with caution.
Piroxicam in small quantities passes into breast milk. Therefore, it is not recommended to use Finalgel during breastfeeding.
Special instructions
For the timely detection of abnormalities in laboratory parameters that may occur during therapy, general clinical blood tests must be repeated every 2 weeks, and biochemical tests every 4 weeks.
With a decrease in platelet counts to less than 50 109 / L. an absolute number of neutrophils less than 0.75 109 / l, a temporary dose reduction of 2 times and a repeat analysis in 1-2 weeks is recommended. If changes persist, treatment is recommended to be discontinued.
When reducing the platelet count to less than 25 109 / L, the absolute number of neutrophils is less than 0.50 109 / L, treatment is recommended to be discontinued.
In the case of the development of immediate-type hypersensitivity reactions (urticaria, angioedema, bronchospasm, anaphylaxis), the drug is canceled and the appropriate drug therapy is immediately prescribed. Transient skin rash does not require discontinuation of therapy.
In the event of signs of impaired liver function, it is necessary to carefully monitor the patient. With the progression of symptoms, the drug should be discontinued.
With mild to moderate impaired renal function, their functional state must be carefully monitored.
Use with caution in patients with severe chronic diseases such as COPD, diabetes mellitus with a tendency to ketoacidosis in patients with bleeding disorders expressed by myelosuppression. In patients receiving Reaferon-EC for a long time, pneumopitis and pneumonia are rarely observed. Timely cancellation of interferon alfa and the appointment of glucocorticosteroid therapy contribute to the relief of pulmonary syndromes.
In patients with thyroid disease, before starting treatment, it is necessary to determine the concentration of thyroid hormone, it is recommended to monitor its level at least 1 time in 6 months. If there is a violation of the thyroid gland function or a deterioration in the course of existing diseases that are not amenable to adequate medical correction, it is necessary to cancel the drug.
In the event of changes in the mental sphere and / or central nervous system, including the development of depression, it is recommended that the psychiatrist be observed during the treatment period, as well as within 6 months after its completion. These disorders are usually rapidly reversible after discontinuation of therapy, however, in some cases, up to 3 weeks are necessary for their complete reverse development. If the symptoms of a mental disorder do not regress or worsen, suicidal thoughts or aggressive behavior directed at other people appear, it is recommended to discontinue treatment with Reaferon-EU and consult a psychiatrist. Suicidal thoughts and attempts are more often observed in children, primarily adolescents (2.4%), than in adults (1%). If therapy using interferon alfa-2b is deemed necessary in adult patients with serious mental disorders (including a history), it should be started, only if appropriate individual screening and psychiatric therapy is performed. The use of interferon alfa-2b in children and adolescents with serious mental disorders (including a history) is contraindicated.
With prolonged use, usually after several months of treatment, visual disturbances are possible. Before starting therapy, an ophthalmic examination is recommended. In case of complaints of any ophthalmic disorders, an immediate consultation with an oculist is necessary. Patients with diseases in which changes in the retina may occur, for example, diabetes mellitus or arterial hypertension, must undergo an ophthalmological examination at least 1 time in 6 months. If visual disturbances occur or worsen, consideration should be given to discontinuing Reaferon-EU therapy.
In elderly patients receiving the drug in high doses, impaired consciousness, coma, convulsions, encephalopathy are possible. In the event of the development of such disorders and the ineffectiveness of dose reduction, treatment should be discontinued.
Patients with diseases of the cardiovascular system and / or advanced oncological diseases require careful observation and monitoring of the ECG. With the development of arterial hyiotension, it is recommended to provide adequate hydration and appropriate therapy.
In patients after transplantation (for example, a kidney or bone marrow), drug immunosuppression may be less effective, because interferon has a stimulating effect on the immune system.
With prolonged use, the preparation of interferon alfa may cause antibodies to interferon in individuals. Usually, antibody titers are low, their appearance does not reduce the effectiveness of treatment.
Use with caution in patients with a predisposition to autoimmune diseases. When symptoms of an autoimmune disease appear, a thorough examination should be carried out and the possibility of continuing treatment with interferon should be assessed. Rarely, interferon alfa therapy is associated with the onset or exacerbation of psoriasis, sarcoidosis.
Influence on the ability to drive vehicles and mechanisms
During the period of use of the drug, patients experiencing fatigue, drowsiness or disorientation should refrain from engaging in potentially dangerous activities that require an increased concentration of attention and speed of psychomotor reactions.
Composition
1 amp. – human recombinant interferon alfa-2b 3,000,000 IU
Excipients: albumin, solution for infusion 10% – 4.5 mg, sodium chloride – 8.52 mg, sodium hydrogen phosphate dodecahydrate – 3.34 mg, sodium dihydrogen phosphate dihydrate – 0.49 mg.
Dosage and administration
The drug is used intramuscularly, subcutaneously, in or under the lesion, subconjunctivally and locally. Immediately before use, the contents of the ampoule or vial are dissolved in water for d / u or 0.9% sodium chloride solution (in 1 ml for i / m, subcutaneous administration and focus, in 5 ml for subconjunctival and local injection). The solution of the drug should be colorless, transparent or with weak opalescence, without sediment and impurities. Dissolution time should be about 3 minutes.
IM and s / d administration of
In acute viral hepatitis B, the drug is administered at 1 million ME 2. times / day for 5-6 days, then the dose is reduced to 1 million ME / day and administered for another 5 days. If necessary (after control biochemical blood tests), the course of treatment can be continued by 1 million. ME 2 times a week for 2 weeks. The course dose is 15-21 million ME.
In acute protracted and chronic viral hepatitis B with the exception of the delta agent and without signs of cirrhosis, the drug is administered 1 million ME 2 times a week for 1-2 months. If there is no effect, treatment should be extended to 3-6 months or after the end of 1-2 months of treatment, 2-3 similar courses should be taken with an interval of 1-6 months.
In chronic viral hepatitis B with a delta agent without signs of liver cirrhosis, the drug is administered at 500 thousand – 1 million ME / day 2 times a week for 1 month. A second course of treatment after 1-6 months.
In chronic viral hepatitis B with a delta agent and signs of cirrhosis, the drug is administered at 250-500 thousand ME / day 2 times a week for 1 month. When signs of decompensation appear, similar courses are repeated at intervals of at least 2 months.
In acute protracted and chronic active hepatitis C without signs of liver cirrhosis, the drug is administered at 3 million ME 3 times a week for 6-8 months. In the absence of effect, treatment should be extended to 12 months. A second course of treatment after 3-6 months.
In kidney cancer, the drug is used in 3 million ME daily for 10 days. Repeated courses of treatment (3-9 or more) are carried out at intervals of 3 weeks. The total amount of the drug is from 120 million ME to 300 million ME or more.
With hairy cell leukemia, the drug is administered daily at 3-6 million ME for 2 months. After normalization of the clinical blood test, the daily dose of the drug is reduced to 1-2 million ME. Then prescribe maintenance therapy of 3 million. ME 2 times a week for 6-7 weeks. The total amount of the drug is 420-600 million ME or more.
In acute lymphoblastic leukemia in children in remission after induction chemotherapy (at 4-5 months of remission) – 1 million ME1 times a week for 6 months, then 1 time in 2 weeks for 24 months. At the same time, maintenance chemotherapy is performed.
In malignant lymphomas and Kaposi s sarcoma, the drug is administered at 3 million ME / day daily for 10 days in combination with cytostatics (Prospidium chloride, cyclophosphamide) and GCS. In the tumor stage of fungoid mycosis, primary reticulosis and reticulosarcomatosis, it is advisable to alternate the intramuscular injection of the drug with 3 million ME and the intrafocal – 2 million ME for 10 days.
In patients with the erythrodermic stage of mushroom mycosis, when the temperature rises above 39 ° C and in case of exacerbation of the process, the drug should be discontinued. With insufficient therapeutic effect, after 10-14 days, a second course of treatment is prescribed. After achieving the clinical effect, maintenance therapy is prescribed at 3 million ME once a week for 6-7 weeks.
In chronic myeloid leukemia, the drug is administered at 3 million ME daily or 6 million ME through laziness. The duration of treatment is from 10 weeks to 6 months.
With histiocytosis from Langerhans cells, the drug is administered at 3 million ME daily for 1 month. Repeated courses at 1-2-month intervals for 1-3 years.
In case of sublepkemic myelosis and essential thrombocythemia for the correction of gynerthrombocytosis – 1 million ME daily or after 1 day for 20 days.
In case of respiratory laryngeal papillomatosis, the drug is administered at 100-150 thousand ME per kilogram of body weight daily for 45-50 days, then at the same dosage 3 times per pedal for 1 month. The second and third courses are carried out with an interval of 2-6 months.
In individuals with a high pyrogenic reaction (39 ° C and above), the simultaneous use of paracetamol or indomethacin is recommended.
Perifocal administration of
In case of basal cell and squamous cell carcinoma, keratoacanthoma, the drug is administered under the lesion of 1 million ME 1 time / day daily for 10 days. In the case of severe local inflammatory reactions, administration under the lesion is carried out after 1-2 days. At the end of the course, if necessary, conduct cryodestruction.
Subconjunctival administration of
In stromal keratitis and keratoiridocyclitis, subconjunctival injections of the drug are prescribed in a dose of 60 thousand ME in a volume of 0.5 ml daily or every other day, depending on the severity of the process. Injections are performed under local anesthesia with 0.5% dicaine solution. The course of treatment is from 15 to 25 injections.
Topical application
For topical application, the contents of the ampoule of the drug are dissolved in 5.0 ml of a 0.9% solution of sodium chloride d / i. In the case of storing a solution of the drug, it is necessary, following the rules of asepsis and antiseptics, transfer the contents of the ampoule to a sterile vial and store the solution in the refrigerator at 4-10 ° C for no more than 12 hours.
In conjunctivitis and superficial keratitis, 2 drops of solution are applied to the conjunctiva of the affected eye 6-8 times / day. As the inflammatory phenomena disappear, the number of installations is reduced to 3-4. The course of treatment is 2 weeks.
Side effects
The frequency of adverse reactions is given in accordance with the WHO classification: very frequent – 1/10, frequent – more than 1/100, but less than 1/10, infrequent – more than 1/1000, but less than 1/100. rare – more than 1/10000, but less than 1/1000 and very rare when occurring less than 1/10000, including individual messages.
When using Reaferon-EU (in clinical trials and outside of clinical trials) the following undesirable effects were observed:
Often with parenteral administration of the drug there is a flu-like syndrome (chills. fever, asthenia, fatigue, fatigue, myalgia, arthralgia, headaches), partially stopped by paracetamol, indomethacin. As a rule, the flu-like syndrome appears at the beginning of treatment and decreases with continuation.
From the cardiovascular system: rarely – arrhythmias, transient reversible cardiomyopathy very rarely – arterial hypotension, myocardial infarction.
From the digestive system: rarely – dry mouth, nausea, abdominal pain, dyspepsia, appetite disturbances, weight loss, vomiting, diarrhea is very rare – pancreatitis, hepatotoxicity.
From the side of the central nervous system: rarely – irritability, nervousness, depression, asthenia, insomnia, anxiety, impaired ability to concentrate, suicidal thoughts, aggressiveness very rarely – neuropathy, psychosis.
From the skin: rarely – skin expulsion and itching. increased sweating, hair loss. When introduced into the lesion or under the lesion rarely – a local inflammatory reaction. These side effects are usually not an obstacle to the continued use of the drug.
From the endocrine system: against the background of prolonged use of the drug, changes in the thyroid gland are possible. Very rarely – diabetes.
On the part of laboratory parameters: when using the drug, abnormalities in laboratory parameters are possible, manifested by leukopenia, lymphopeeia, thrombocytopenia, anemia, increased activity of alanine aminotransferase, alkaline phosphatase, creatinine, urea concentration. As a rule, these changes are usually minor, asymptomatic and reversible.
From the musculoskeletal system: rarely – rhabdomyolysis, leg cramps, back pain, myositis, myalgia.
From the respiratory system: rarely – pharyngitis, cough, dyspnoea, pneumonia.
From the urinary system: very rarely – renal failure.
From the side of the immune system: rarely – autoimmune pathology (vasculitis, rheumatoid arthritis, lupus-like syndrome) very rarely – sarcoidosis, angioedema, anaphylaxis, swelling of the face.
From the side of the organs of vision: with topical administration of the drug on the mucous membrane of the eye, hyperemia, single follicles, swelling of the conjunctiva of the lower arch are possible. Rarely, hemorrhages in the retina, focal changes in the fundus, thrombosis of arteries and veins of the retina, decreased visual acuity, optic neuritis, edema of the optic nerve head.
On the part of the hearing organs: rarely – hearing impairment.
In case of pronounced local and general adverse reactions, the drug should be discontinued.
Drug interaction
Interferon alfa-2b is able to reduce the activity of isoenzymes of cytochrome P450 and, therefore, affect the metabolism of cimetidine, phenytoin, chimetry, theophylline. diazepam, propranolol, warfarin. some cytostatics. May enhance the neurotoxic, myelotoxic, or cardiotoxic effects of drugs previously prescribed or at the same time. Avoid co-administration with drugs that depress the central nervous system. immunosuppressive drugs (including oral and parenteral forms of corticosteroids).
Interferons can affect oxidative metabolic processes. This should be taken into account with simultaneous use with drugs metabolizable by oxidation (including with xanthine derivatives – aminophylline and theophylline). With the simultaneous use of Reaferon-EC with theophylline, it is necessary to control the concentration of theophylline in the blood serum and, if necessary, adjust the dosage regimen.
With the combined use of Reaferon-EC and hydroxyurea, the incidence of cutaneous vasculitis may increase.
Drinking alcohol during treatment is not recommended.
Overdose of
No overdose was observed. Given that the active substance is interferon alfa-2b, the overdose may increase the severity of dose-dependent side effects.
Treatment: withdrawal of the drug, if necessary, conduct symptomatic therapy.
Storage Conditions
The product should be stored in a dark place and out of the reach of children at a temperature not exceeding 8 ° C.
Shelf life
3 years.
An expired drug is not to be used.
Deystvuyushtee substance
Interferon alyfa-2b
Terms and conditions
For prescription
dosage form
injection