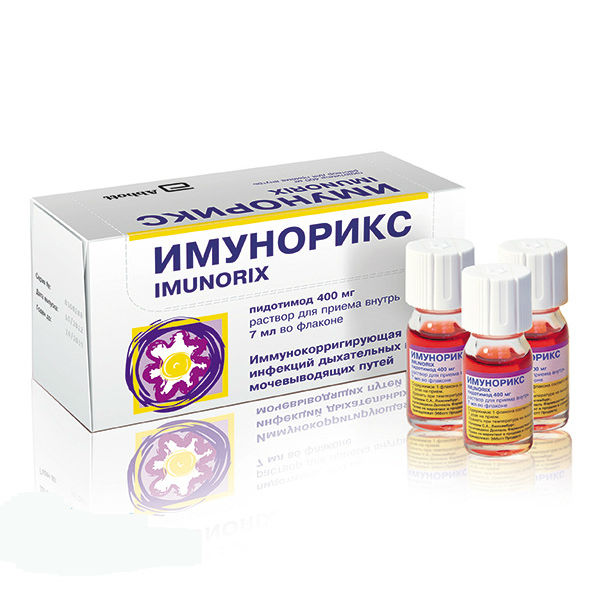
Description
Release form
Red-violet oral solution, clear, with the smell of wild berries.
Packaging
7 ml – single-dose glass vials (10) – packs of cardboard.
Pharmacological action
Immunostimulating drug.
Pidotimod stimulates and regulates cellular immunity. It induces the maturation and formation of immunocompetent T-lymphocytes in case of their insufficiency, which are assigned the role of coordinators of specific immunity in physiological conditions due to partial replacement or enhancement of the functions of the thymus gland. In addition, pidotimod stimulates macrophages, whose main function is to capture the antigen and its presentation on the cell membrane in combination with histocompatibility antigens. The body’s ability to counter infectious agents is expressed in effective specific immune, cellular and antigen-antibody protective responses.
Pidotimod exerts therapeutic effects through an immunostimulating effect on innate immunity and antibody production, on cellular immunity and on cytokine production.
Pidotimod increases the production of superoxide anions, tumor necrosis factor-alpha, NO (bactericidal action), as well as chemotaxis and, accordingly, phagocytosis. The drug also increases the cytotoxic activity of natural killers.
Pidotimod enhances the functional activity of T and B lymphocytes, increases the stimulation of the antigen-antibody reaction and prevents the development of apoptosis induced by dexamethasone, 12-O-tetradecanoylphorbol-13-acetate and calcium ionophore A-23I87.
Pidotimod increases the content of interleukin-2 (IL-2) in old rats and the expression of the IL-2 gene in rat spleen. In particular, it was shown that pidotimod has an immunostimulating effect in cases of insufficiency of the immune system, as well as its functioning at the physiological level.
Pharmacokinetics
Absorption
When administered, absorption is high. Bioavailability is 45%.
The speed and degree of absorption of pidotimod is significantly reduced while taking with food. Oral bioavailability with food is reduced to 50%, serum Cmax is reached 2 hours later compared with fasting.
Distribution and metabolism
Pidotimod does not bind to plasma proteins and is not metabolized.
Excretion of
T1 / 2 is 4 hours. The drug is excreted unchanged in the urine (95% of the administered iv dose).
Pharmacokinetics in special patient groups
Pharmacokinetic studies in elderly patients did not reveal differences from pharmacokinetics in young patients.
T1 / 2 increases with renal failure. Nevertheless, in severe renal failure (plasma creatinine content of 5 mg / dl) T1 / 2 pidotimoda does not exceed 8-9 hours. patients take the drug every 12 hours or 24 hours, there is no risk of cumulation in renal failure.
Studies in liver failure have not been conducted since the drug is almost completely excreted in the urine unchanged.
Indications
Immunostimulating therapy for disorders in the cellular immunity:
for upper and lower respiratory tract infections
for urinary tract infections.
It is used both to prevent exacerbations and to reduce the duration and severity of individual episodes, and as an adjuvant for antibiotic therapy of acute infections.
Contraindications
children under 3 years of age
fructose intolerance
hypersensitivity to pidotimod or to any other component of the drug.
Caution is advised to use the drug in patients with a history of hyperimmunoglobulinemia E, allergic reactions, or a history of atopic dermatitis.
Use during pregnancy and lactation
Experience with pidotimod during pregnancy is absent or limited (less than 300 pregnancy outcomes). In experimental studies in animals, no direct or indirect adverse effects on reproductive function were revealed.
Immunix should be avoided in the first trimester of pregnancy.
There is no data on the allocation of pidotimod or its metabolites with breast milk. Breastfeeding should be stopped during the period of treatment with the drug in order to avoid the effects of the active substance on the baby.
Composition
1 bottle contains:
Active ingredient:
Pidotimod 400.0 mg
Excipients:
Sodium chloride 5.6 mg, sodium
0 mg,
disodium edetate 3.5 mg,
trometamol qs up to pH 6.5 (which corresponds to ~ 193 mg per 7.0 ml),
me sodium tyl parahydroxybenzoate 10.3 mg,
sodium propyl parahydroxybenzoate 1.6 mg,
sorbitol 70% 2500 mg,
fruit flavor (with the smell of wild berries) 21.0 mg,
dye anthocyanin 5.6 mg,
dye crimson [Ponso 4R] 0.5 mg,
purified water to 7.0 ml.
Dosage and administration of
The drug is taken orally. Since food affects the absorption of Imunorix, it should be taken outside of meals.
Adults in the acute phase of the disease are prescribed 800 mg (2 bottles) 2 times / day 2 hours before or 2 hours after eating for 2 weeks as maintenance therapy – 800 mg (2 bottles) 1 time / day within 60 days.
For prophylaxis, 800 mg (2 vials) should be taken 1 time / day 2 hours before or 2 hours after a meal for 60 days.
Children older than 3 years in the acute phase of the disease are prescribed 400 mg (1 bottle) 2 times / day 2 hours before or 2 hours after eating for 2 weeks as maintenance therapy – 400 mg (1 bottle) 1 time / day for 60 days.
For prophylaxis, 400 mg (1 bottle) is prescribed 1 time / day 2 hours before or 2 hours after a meal for 60 days.
Drug Interaction
Pidotimod does not bind to plasma proteins and is not metabolized, so pharmacokinetic interaction is not expected.
The drug can affect the effectiveness of drugs that suppress or stimulate the functional activity of lymphocytes or affect the activity of the immune system.
In experimental studies in animals, when combining pidotimod with other commonly used drugs (eg, hypoglycemic agents (tolbutamide), antiepileptic drugs (phenobarbital), antihypertensive agents (nifedipine, captopril, chlorophyll, attenopril, chlorophyll), anticoagulants (warfarin), NSAIDs (indomethacin), analgesics (acetylsalicylic acid) or antipyretics (paracetamol)) did not show any undesirable interaction.
Overdose
No cases of overdose and use of the drug have been reported by destination.
Treatment: There is no specific data on the treatment of overdosage with the drug. In the event of an overdose, the patient should be consulted immediately. Symptomatic and supportive therapy is recommended. Careful observation should continue until the patient recovers.
Storage conditions
The drug should be stored out of the reach of children at a temperature not exceeding 30 ° C.
The Expiration of
is 3 years.
Deystvuyuschee substances
Pydotymod
Dosage form
Dosage form
oral solution
Doppely Farmatseutitsi, Italy