Description
Latin name
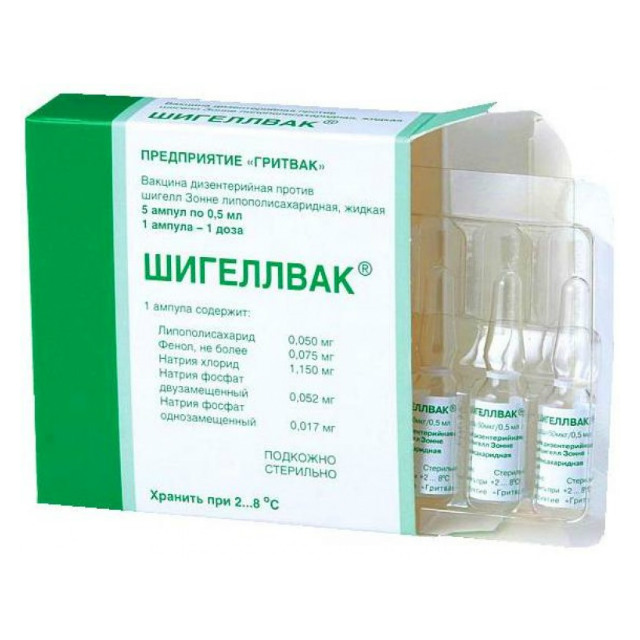
SHIGELAT
Release form
Solution for intramuscular and subcutaneous administration
Pharmacological action
Forms specific resistance to Shigella sonnei, stimulates the appearance of specific antibodies, which provide in 2-3 weeks. immunity to infection for 1 year.
Indications
Dysentery Sonne (prevention).
Contraindications
Hypersensitivity (including the previous administration of dysentery vaccine), an acute period of infectious non-infectious diseases, exacerbations of chronic diseases, the period of convalescence or remission for 1 month.
after the acute period of the disease, pregnancy, children’s age (up to 3 years).
Special instructions
Prophylactic vaccination is recommended for workers of infectious hospitals and bacteriological laboratories
people involved in public catering and public amenities: children attending childcare facilities and leaving for health camps
people departing to high seasons a seasonal increase in the incidence of this infection.
According to epidemiological indications, vaccinations are carried out in case of an epidemic or are more likely to occur (natural disasters, major accidents on the water supply and sewer network).
In order to identify contraindications on the day of vaccination, a complete medical history should be collected and thermometry performed, if necessary, necessary laboratory tests are carried out.
Before vaccination, evaluate the integrity of the ampoule and packaging, the state of the physical properties of the drug, check its Expiration.
Composition
Liposaccharide solution,
extracted from Shigella sonnei culture,
purified by enzymatic and physico-chemical methods.
Dosage and administration
Adults and children 3 years of age and older – sc (deep) or im in the outer surface of the upper third of the shoulder – 50 mcg (0.5 ml) once.
If necessary, revaccinate in the same dose every year.
Opening of ampoules and the procedure for administering the drug is carried out in strict compliance with the rules of asepsis and antiseptics.
An open ampoule preparation should be used immediately.
The vaccine is recorded in the prescribed registration forms with the name of the drug and the date of vaccination, dose, manufacturer, lot number and reaction to the vaccine.
Side effects
Rarely (during the first days after immunization) – hyperthermia (in 3-5% of cases – subfibrillation within 24-48 hours), headache, local reactions (hyperemia, pain at the injection site).
Drug interactions
Vaccination can be carried out on the same day as other preventive vaccinations with inactivated drugs or with an interval after the last vaccination of at least 1 month.
Storage conditions
Store at 2-8 deg. C.
Deystvuyuschee substances
Vaccine for Prevention dysentery
Terms and conditions
prescription
dosage form
solution Injection
Gritvak, Russia