Description
Release form
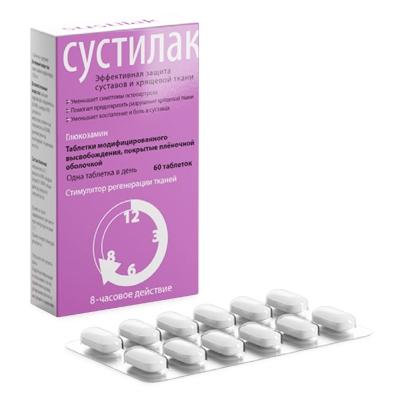
Modified release tablets, film coated white or almost white, oval.
Packing
15 pcs. – blister packagings (4) – packs of cardboard.
Pharmacological action
Clinical and pharmacological group: A drug that affects the metabolism of cartilage.
proteoglycan biosynthesis stimulator Pharmacotherapeutic group: Tissue regeneration
stimulator Pharmacological action of
Glucosamine helps prevent cartilage destruction, stimulates the restoration of cartilage tissue, has anti-inflammatory and analgesic effects, and normalizes the production of intraarticular fluid. Improves joint mobility, reduces the need for NSAIDs.
Pharmacokinetics
Absorption and distribution of
After oral administration, it is rapidly and completely absorbed into the gastrointestinal tract, the prolonged form ensures continuous release of the active substance and its uniform absorption, the bioavailability of glucosamine after oral administration is 25-26% (it undergoes the effect of the first passage through the liver). After distribution in the tissues, the highest concentrations are observed in the liver, kidneys and cartilage.
Metabolism and excretion
Metabolized to water, carbon dioxide and urea. It is excreted mainly by the kidneys.
Indications
– Osteoarthritis of the peripheral joints and spine.
Contraindications
– severe renal dysfunction
– pregnancy
– lactation period
– age under 18 years (efficacy and safety have not been established)
– hypersensitivity to glucosamine and other components of the drug.
Precautions should be prescribed for bronchial asthma, diabetes mellitus, intolerance to seafood (shrimp, shellfish).
Special instructions
The risk of allergic reactions increases with intolerance to seafood.
Effect on driving ability and driving mechanisms
There are no restrictions regarding driving
Composition
1 tab.
glucosamine hydrochloride 1500 mg
Excipients: calcium hydrogen phosphate – 62 mg, hypromellose (K200M) – 40 mg, povidone K90 – 40 mg, microcrystalline cellulose – 83 mg, hypromellose (K100M) – 80 mg, silicon colloidal dioxide – 5 mg, stearin acid – 40 mg.
Shell composition: hypromellose E-5 – 20 mg, macrogol-6000 – 6 mg, white opadray (titanium dioxide, macrogol, hypromellose) – 11 mg.
Dosage and administration of
The drug is taken orally. The tablets are swallowed whole washed down with water.
Recommended dose – 1 tab. 1 time / day, for 6-12 weeks. On the recommendation of a doctor, the treatment is repeated at intervals of 2 months. A stable therapeutic effect is achieved when taking the drug for 6 months.
Side effects
Tolerance to the drug is good, in some cases possible: gastrointestinal tract dysfunction (epigastric pain, flatulence, constipation or diarrhea, nausea, skin allergic reactions (urticaria, itchy skin).
Drug interaction
Increases the absorption of tetracyclines, reduces the effect of semisynthetic penicillins, chloramphenicol.
The drug is compatible with paracetamol, NSAIDs and corticosteroids. When combined with NSAIDs, it enhances the anti-inflammatory and analgesic effects of the latter.
Overdose
Overdose is unknown.
Treatment: gastric lavage, symptomatic therapy.
Storage Conditions
Keep this medicine out of the reach of children, dry, protected from light at a temperature not exceeding 25 ° C.
Terms leave through pharmacies
without prescription
Dosage form
tablet prolong.
Selebriti Biopharma Ltd, India