Description
Latin name
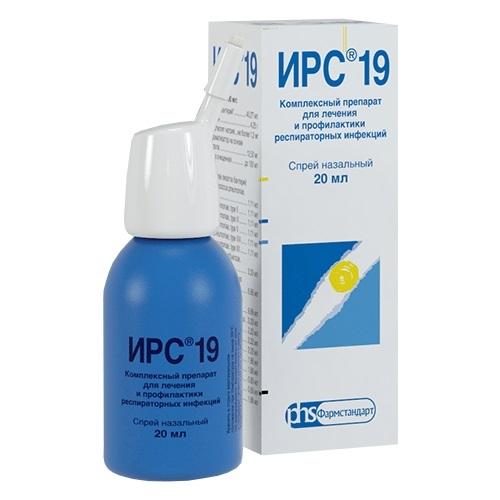
IRS 19
Release form
Nasal spray in the form of a transparent, colorless or yellowish liquid with a faint specific odor.
Packaging
20 ml – aerosol glass containers (1) with a continuous valve and nozzle – cardboard packs.
Pharmacological action
Immunostimulating drug based on bacterial lysates. IRS 19 enhances specific and non-specific immunity.
When spraying IRS 19, a fine aerosol is formed that covers the nasal mucosa, which leads to the rapid development of a local immune response. Specific protection is due to locally generated antibodies of the class A secretory immunoglobulin type A (IgA), which prevent the fixation and reproduction of infectious agents on the mucosa. Non-specific immunoprotection is manifested in an increase in the phagocytic activity of macrophages, increase in lysozyme content.
Indications
Adults and children 3 months of age and older:
prevention of chronic diseases of the upper respiratory tract and bronchi
treatment of acute and chronic diseases of the upper respiratory tract and bronchi, such as rhinitis, sinusitis, laryngitis, pharyngitis, tonsillitis, tracheitis, bronchitis and other restoration of local immunity after the transferred flu or other viral infections
preparation for planned surgical intervention on the ENT organs and the postoperative period.
Contraindications
autoimmune diseases
hypersensitivity to the components of the drug.
Pregnancy and lactation
There is insufficient evidence of the potential for teratogenicity or toxic effects on the fetus during pregnancy. Therefore, the use of the drug IRS 19 during pregnancy is not recommended.
Composition
100 ml
bacterial lysates 43.27 ml,
incl. Streptococcus pneumoniae type I 1.11 ml
Streptococcus pneumoniae type II 1. methylanthranilate, limonene, geranyl acetate, linalyl acetate, diethylene glycol monoethyl ether, phenylethyl alcohol) – 12.5 mg, purified water – up to 100 ml.
Dosage and administration
The drug is administered intranasally by aerosol administration of 1 dose (1 dose = 1 short press of the atomizer).
For the purpose of prevention, adults and children from 3 months of age are given 1 dose of the drug in each nostril 2 times / day for 2 weeks (it is recommended to start the course of treatment 2-3 weeks before the expected rise in the incidence).
For the treatment of acute and chronic diseases of the upper respiratory tract and bronchi, children from 3 months to 3 years of age are prescribed 1 dose of the drug in each nostril 2 times / day after preliminary release from the mucous discharge until the symptoms of infection disappear for children older than 3 years and adults – 1 dose of the drug in each nostril from 2 to 5 times / day until the symptoms of infection disappear.
To restore local immunity after the transferred flu and other respiratory viral infections, children and adults are prescribed 1 dose of the drug in each nostril 2 times / day for 2 weeks.
In preparation for the planned surgical intervention and in the postoperative period, adults and children are prescribed 1 dose of the drug in each nostril 2 times / day for 2 weeks (it is recommended to start the course of treatment 1 week before the planned surgical intervention).
Terms of use of the drug
For the correct functioning of the aerosol can, put the nozzle on the can, center it and gently, without pressure, press it. After that, the device is ready for use.
When injecting the drug, the vial should be in a strictly upright position, the patient should not throw his head back.
If you tilt the cylinder during injection, the propellant will leak out in a few seconds and the device will become unusable.
With regular use of the drug, it is not recommended to remove the nozzle from the vial.
If the preparation is left for a long time without use, a drop of liquid may evaporate and the crystals formed will clog the nozzle outlet. This happens most often when the nozzle is removed and placed in the package with the upper end down next to the cylinder, without first rinsing and not drying it. If the nozzle becomes clogged, press several times in a row, so that the liquid can pass under the influence of excessive pressure, if there is no effect, lower the nozzle for several minutes in warm water.
Side effects
Dermatological reactions: rarely – erythema and eczema-like reactions in isolated cases – thrombocytopenic purpura and erythema nodosum.
Allergic reactions: rarely – urticaria, angioedema.
From the respiratory system: rarely – asthma attacks and cough, at the beginning of treatment – rhinopharyngitis, sinusitis, laryngitis, bronchitis.
From the digestive system: rarely (at the beginning of treatment) – nausea, vomiting, abdominal pain, diarrhea.
Other: rarely (at the beginning of treatment) – an increase in body temperature (> 39 ° C) for no apparent reason.
Side effects can be either related or not related to the effect of the drug. If the above symptoms appear, it is recommended to consult a doctor.
Drug Interaction
Cases of negative interaction of IRS 19 with other drugs are unknown.
In the event of clinical symptoms of bacterial infection, antibiotics may be prescribed against continued use of IRS 19.
Overdose
The overdose of IRS 19 is unknown.
Storage conditions
The drug should be stored out of the reach of children, in a strictly vertical position at a temperature not exceeding 25 ° C, do not freeze.
The bottle should be protected from heat above 50 ° C and from direct sunlight, do not pierce the bottle, do not burn, even if it is empty.
The Expiration of
is 3 years.
pharmacies over-the-counter conditions
Dosage form
Dosage form
nasal spray