Description
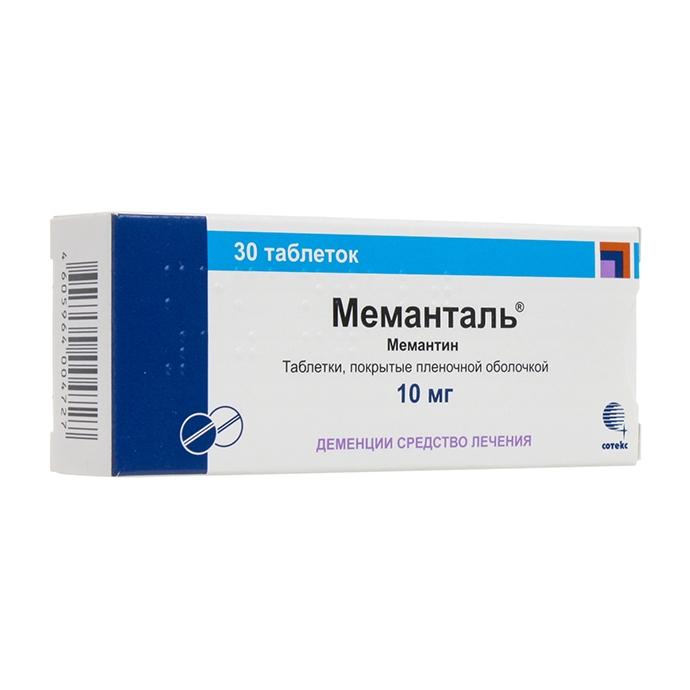
Release form
Tablets: round, biconvex, coated with a white film coat, with a wide risk side and marked with “M9MN” and “10” on the other.
Pharmacological action
Pharmacotherapeutic group:
dementia treatment cure
ATX code: N06DX01
Pharmacological properties
Pharmacodynamics
Has a nootropic, cerebro-vasodilating, antihypoxic and psychoactive effect. The adamantane derivative is close to amantadine in chemical structure and pharmacological properties. It blocks glutamate N-methyl-D-aspartate receptors (NMDA receptors) (including in the substantia nigra), thereby reducing the excessive stimulating effect of cortical glutamate neurons on neostratum, which develops against the background of insufficient dopamine secretion. Reducing the flow of ionized calcium into neurons, reduces the possibility of their destruction. To a greater extent affects stiffness (rigidity and bradykinesia). Improves weakened memory, concentration of attention, reduces fatigue and symptoms of depression, reduces spasticity caused by diseases and brain damage.
Pharmacokinetics
After oral administration, memantine is rapidly and completely absorbed from the gastrointestinal tract. The maximum concentration in blood plasma is reached within 2-6 hours.
With normal renal function, no cumulation of memantine was noted.
Withdrawal is biphasic. The elimination half-life averages 4 9 hours in the first phase and 40 65 hours in the second phase. About 80% of memantine is excreted unchanged. Metabolites do not have their own pharmacological activity. It is excreted in the urine. With an alkaline reaction of urine, excretion slows down.
Indications
Moderate to severe dementia in Alzheimer’s disease.
Contraindications
Individual hypersensitivity to the drug, severe renal impairment (creatinine clearance (CC) less than 5-29 ml / min), severe liver failure, pregnancy, breastfeeding, children under 18 years of age (efficacy and safety not established) lactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption (the drug contains lactose monohydrate).
With caution,
is prescribed for patients with thyrotoxicosis, epilepsy, seizures (including a history of) the simultaneous use of NMDA receptor antagonists (amantadine, ketamine, dextromethorphan), the presence of factors that increase the pH of urine (a sharp change in diet, abundant alkaline buffers) severe urinary tract infections, a history of myocardial infarction, heart failure (NYHA class III-IV functional class), uncontrolled hypertension, renal and liver failure.
Pregnancy and lactation
There is no data on the use of memantine in pregnant women . Memantine should only be used if the expected benefit to the mother outweighs the potential risk to the fetus.
There is no data on the penetration of memantine into breast milk. Given the lipophilic structure of the active substance of the drug, we can assume that that memantine can pass into breast milk, and therefore it is recommended to stop breastfeeding while taking the drug.
Special instructions
Caution is prescribed for patients with thyrotoxicosis, epilepsy, seizures (including a history of) the simultaneous use of NMDA receptor antagonists (amantadine, ketamine, dextromethorphan), the presence of factors that increase the pH of urine (a sharp change in dietary intake) gastric buffers), severe urinary tract infections, a history of myocardial infarction, heart failure (NYHA class III-IV functional class), uncontrolled hypertension, renal and hepatic insufficiency accuracy.
Effects on ability to drive vehicles and control mechanisms
Patients with Alzheimer’s disease at the stage of moderate or severe dementia usually have impaired ability to drive vehicles and manage complex mechanisms. In addition, memantine can cause a change in the reaction rate, so patients need to refrain from driving or working with complex mechanisms.
Composition
1 film-coated tablet contains:
active substance: memantine hydrochloride 10.00 mg
excipients:
core tablets – lactose monohydrate 149.75 mg, microcrystalline cellulose 27.10 mg, talc 11.1 , colloidal silicon dioxide 1.25 mg, magnesium stearate 0, 75 mg
shell – Opadray ® white (lactose monohydrate 2.16 mg, hypromellose 1.68 mg, titanium dioxide 1.56 mg, macrogol-4000 0.60 mg) – 6.00 mg.
Dosage and administration of
The drug is taken orally, once a day, always at the same time, regardless of food intake. The dosage regimen is set individually. It is recommended to start treatment with the appointment of the minimum effective dose.
Prescribe the drug for 1 week of therapy (days 1-7) at a dose of 5 mg / day, for 2 weeks (days 8-14) – at a dose of 10 mg / day, for 3 weeks ( days 15-21) – at a dose of 15 mg / day, during the 4th week (days 22-28) – at a dose of 20 mg / day. The maximum daily dose of 20 mg. In patients older than 65 years, as well as patients with CC 50-80 ml / min, dose adjustment is not required. For patients with moderate renal failure (CC 30-49 ml / min), the daily dose is 10 mg. In the future, with good tolerability of the drug for 7 weeks, the dose can be increased to 20 mg according to the standard scheme.
Tablet Dividing Instructions Place the tablet with its rounded side on a hard surface with the notch facing up. Press the index finger and thumb of one of the hands on the opposite sides of the tablet, continue to apply pressure with your fingers until the tablet breaks into two.
Side effects
The frequency of adverse reactions was classified as follows: very often (? 1/10), often (? 1/100, <1/10), infrequently (? 1/1000, <1/100), rarely ( ? 1/10000, <1/1000), very rarely (<1/10000), the frequency has not been established (there are currently no data on the prevalence of adverse reactions). From the central nervous system: often – headache, drowsiness, dizziness is rare – confusion, hallucinations (mainly in patients with Alzheimer’s disease at the stage of severe dementia), gait disturbance is very rare – convulsions frequency is not established – psychotic reactions. From the digestive system: often – constipation rarely – nausea, vomiting frequency is not established – pancreatitis. From the cardiovascular system: rarely – arterial hypertension, venous thrombosis / thromboembolism. Other: rarely – fatigue, fungal infections. There are separate reports of the occurrence of these adverse reactions when using memantine in clinical practice: Dizziness, drowsiness, increased irritability, increased fatigue, anxiety, increased intracranial pressure, nausea, hallucinations, headache, impaired consciousness, muscle hypertonia, gait disorders, depression, convulsions, psychotic reactions, suicidal thoughts, constipation, nausea, pancreatitis, candidomycosis, increased blood pressure, vomiting, cystitis, increased libido, venous thrombosis, thromboembolism, allergic reactions. Drug interaction When used simultaneously with levodopa drugs, antagonists of dopamine receptors, m-anticholinergic agents, the effect of the latter can be enhanced. With simultaneous use with barbiturates and antipsychotics, the effect of the latter may decrease. When used together, it can change (increase or decrease) the effect of dantrolene or baclofen, so the doses of the drugs should be selected individually. Concomitant use with amantadine, ketamine, phenytoin, and dextromethorphan should be avoided because of the increased risk of developing psychosis. Possible increase in plasma concentrations of cimetidine, ranitadine, procainamide, quinidine, quinine and nicotine when taken with memantine. May decrease hydrochlorothiazide levels when used together with memantine. Memantine is able to increase the excretion of hydrochlorothiazide. May increase MHO (international normalized ratio) in patients taking oral anticoagulants (warfarin). Concomitant use with antidepressants, selective serotonin reuptake inhibitors, and monoamine oxidase inhibitors requires close monitoring of patients. Overdose Symptoms: dizziness, tremor, agitation, drowsiness, confusion, agitation, stupor, convulsions, psychosis, aggressiveness, hallucinations, vomiting, unsteady gait, diarrhea. Treatment: gastric lavage, intake of activated carbon, symptomatic therapy. There is no specific antidote. Storage Conditions At a temperature not exceeding 25 ° C. Keep out of reach of children! Expiration 3 years. active substance Memantine Terms leave through pharmacies In retseptu lekarstvennaja form tablets